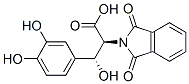
(2S,3R)-3-(3,4-Dihydroxyphenyl)-3-hydroxy-2-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)propionic acid synthesis
- Product Name:(2S,3R)-3-(3,4-Dihydroxyphenyl)-3-hydroxy-2-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)propionic acid
- CAS Number:96561-53-4
- Molecular formula:C17H13NO7
- Molecular Weight:343.29
![(2R,3S)-3-(1,3-Benzodioxol-5-yl)-3-hydroxy-2-[1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl]propionic acid](/CAS/GIF/96561-34-1.gif)
96561-34-1
1 suppliers
inquiry

96561-53-4
5 suppliers
inquiry
Yield:96561-53-4 50 kg
Reaction Conditions:
with aluminum (III) chloride;Octanethiol in dichloromethane at -20 - 15;Large scale;
Steps:
2 Synthesis of L-threo(N-phthaloyl-3-(3,4-dihydroxyphenyl)serine)
Compound 10 (62 kg) wet cake is added to a reactor containing methylene chloride (1240 L) and stirred for 10 min. The mixture is heated to remove methylene chloride and water under azeotropic reflux. After methylene chloride (1550 L) is removed and no water remains in the distillate, the mixture is cooled to 25-30° C. An in-process sample is taken to determine water content (limit Methylene chloride (186 L) is added to another reactor at 25-30° C. An in-process sample is taken to check for water content (limit ≦0.2% w/w). Aluminum chloride (81 kg) is added and the contents are stirred at 25-30° C. for 10-15 min. The mixture is cooled to 10-15° C. and octanethiol (78 kg) is added. The mixture is cooled to -20 to -10° C. The slurry of 10 in methylene chloride controlled at -20 to -7° C. is added to the stirred mixture that is temperature controlled at -15 to -10° C. for 20-30 min. The mixture is heated to 10-15° C. for 1.5-2.5 h. An in-process sample is taken to determine 10 content (limit ≦3.5%). The mixture is further cooled to -20 to -10° C. and then transferred to another reactor containing oxalic acid (62 kg), methylene chloride (186 L), and demineralized water (744 L) while maintaining the temperature below -3° C. to quench the reaction. The quenched material is slowly heated to 25-30° C. and maintained at this temperature for 12 h. Methylene chloride is distilled out at 25-30° C. under vacuum until the mixture volume is reduced to 1364 L. The mixture is centrifuged for 3 h and the wet cake is washed with demineralized water (62 L). The wet cake is added to a reactor containing oxalic acid (2.5 kg) and demineralized water (248 L) and the contents are stirred at 25-30° C. for 2 h to obtain a clear solution. The material is centrifuged for 1 h 15 min to 1 h 30 min and the wet cake is washed twice with demineralized water (186 L). The wet cake is added to a reactor containing demineralized water (248 L) at 25-30° C. and the contents are stirred for 2 h. The material is centrifuged for 1 h 30 min to 2 h and the wet cake is washed twice with demineralized water (186 L). The material is collected and placed into preweighed containers. Expected yield of L-threo(N-phthaloyl-3-(3,4-dihydroxyphenyl)serine (11): 40-50 kg.
References:
US2013/253061,2013,A1 Location in patent:Paragraph 0068-0069
85-41-6
489 suppliers
$9.00/1g

96561-53-4
5 suppliers
inquiry

25543-10-6
5 suppliers
inquiry

96561-53-4
5 suppliers
inquiry

1458647-14-7
0 suppliers
inquiry

96561-53-4
5 suppliers
inquiry