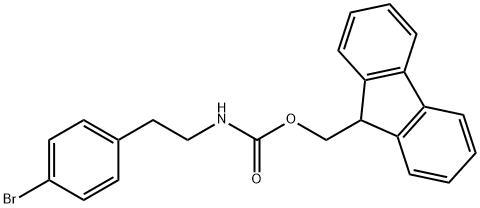
(9H-Fluoren-9-yl)Methyl 4-broMophenethylcarbaMate synthesis
- Product Name:(9H-Fluoren-9-yl)Methyl 4-broMophenethylcarbaMate
- CAS Number:1344158-44-6
- Molecular formula:C23H20BrNO2
- Molecular Weight:422.31
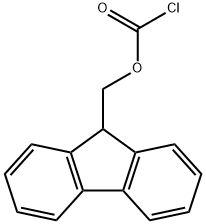
28920-43-6
550 suppliers
$5.00/5g
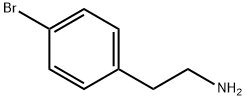
73918-56-6
233 suppliers
$20.00/1g

1344158-44-6
8 suppliers
inquiry
Yield:1344158-44-6 50%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane at 0 - 20; for 20 h;
Steps:
6.6.2.39. [2-(3-Hydroxy-phenyl)-ethyl]-carbamic acid 9H-fluoren-9-ylmethyl ester (28e)
General procedure: A solution of FmocCl (4.3 g, 16.8 mmol) in DCM (20 mL) was added to a stirred solution of 3-(2-amino-ethyl)-phenol 27e (2.0 g, 14.6 mmol) and DIPEA (7.2 mL, 5.6 g, 43.7 mmol) in DCM (30 mL) at 0 °C. After 15 min at 0 °C, the mixture was stirred for 20 h at room temperature. The reaction mixture was diluted with DCM (50 mL), washed successively with a saturated aqueous NaHCO3 solution (1 × 20 mL) and brine (1 × 20 mL). The organic phase was dried over Na2SO4, filtered and concentrated under vacuum. The crude residue was purified by flash column chromatography (DCM/MeOH 1:0 to 95:5) to provide the title compound (1.2 g, 23% yield) as a white solid.
References:
Maillard, Michel C.;Brookfield, Frederick A.;Courtney, Stephen M.;Eustache, Florence M.;Gemkow, Mark J.;Handel, Rebecca K.;Johnson, Laura C.;Johnson, Peter D.;Kerry, Mark A.;Krieger, Florian;Meniconi, Mirco;Mu?oz-Sanjuán, Ignacio;Palfrey, Jordan J.;Park, Hyunsun;Schaertl, Sabine;Taylor, Malcolm G.;Weddell, Derek;Dominguez, Celia [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 19,p. 5833 - 5851] Location in patent:experimental part