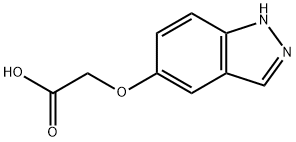
Acetic acid, 2-(1H-indazol-5-yloxy)- synthesis
- Product Name:Acetic acid, 2-(1H-indazol-5-yloxy)-
- CAS Number:30226-16-5
- Molecular formula:C9H8N2O3
- Molecular Weight:192.17
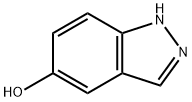
15579-15-4
204 suppliers
$5.00/250mg
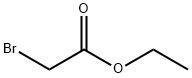
105-36-2
349 suppliers
$5.00/5G

30226-16-5
17 suppliers
inquiry
Yield:30226-16-5 99%
Reaction Conditions:
Stage #1: 5-hydroxy-1H-indazole;ethyl bromoacetatewith potassium carbonate in acetone; for 16 h;Reflux;Inert atmosphere;
Stage #2: with methanol;sodium hydroxide in acetone;Inert atmosphere;
Steps:
1H-Indazol-5-yloxyacetic acid (10s).
A solution of liT-inazol-5-ol (2.8 g, 20.81 mmol), ethyl bromoacetate (2.8 mL, 25.0 mmol) and K2CC>3 (5.8 g, 41.61 mmol) in acetone (30 mL) was stirred and heated at reflux for 16 h. The remaining solids were filtered off, washing with acetone, and the filtrate was evaporated under reduced pressure. 5 M NaOH(aq) (40 mL) and MeOH (20 mL) were added, and the resulting solution stirred at rt for 3 h. Then, the MeOH was removed under reduced pressure and the remaining aqueous solution acidified via addition of 6 M HCl(aq). The aqueous solution was extracted with EtOAc (30 mL c 3) and the combined organic layers washed with brine, dried (MgSCri) and evaporated under reduced pressure to give 10s (4.1 g, 99%) as a brown solid, m.p.: stable under 300 °C; IR (solid) 3339 (O-H + N-H str), 1706 (C=0), 1611, 1511, 1102 cnr1; NMR (300 MHz, DMSO-i) S 12.91 (br s, 2H, OH + NH), 7.93 (d, 7= 1.0 Hz 1H, Ar), 7.45 (dt, J= 9.0, 1.0 Hz, 1H, Ar), 7.10 (d, J= 2.0 Hz, 1H, Ar), 7.03 (dd, J= 9.0, 2.0 Hz, 1H, Ar), 4.64 (s, 2H, OC H2); 13C NMR (75.5 MHz, DMSO-i) d 170.5 (C), 151.8 (C), 135.9 (C), 132.6 (C), 122.8 (CH), 117.9 (CH), 111.1 (CH), 100.9 (CH), 65.3 (CH2).
References:
WO2021/188728,2021,A1 Location in patent:Paragraph 00165; 00166; 00198; 00233