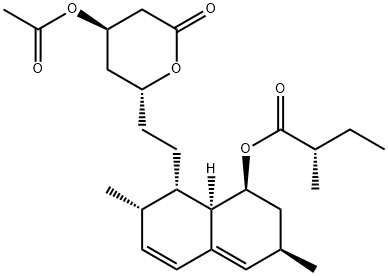
ACETYLLOVASTATIN synthesis
- Product Name:ACETYLLOVASTATIN
- CAS Number:81189-92-6
- Molecular formula:C26H38O6
- Molecular Weight:446.58
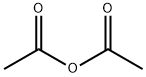
108-24-7
0 suppliers
$14.00/250ML
75330-75-5
568 suppliers
$20.00/5mg

81189-92-6
19 suppliers
inquiry
Yield:81189-92-6 84%
Reaction Conditions:
with pyridine at 20; for 2.5 h;Inert atmosphere;
Steps:
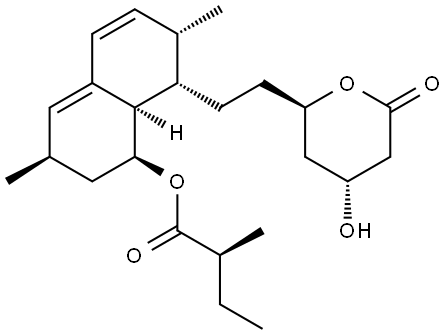
(S)-2-Methyl-butyricacid (3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-acetoxy -6-oxo-tetrahydro-pyran-2yl]-ethyl}-3,7-dimetyl-1,2,3,7,8,8a-hexahydro-naphthalen-1-yl ester (2a)
To a solution of lovastatin (1, 1.00 g, 2.48 mmol) in pyridine (5 mL) was added Ac2O (2.5mL). The resulting solution was stirred under N2 at RT for 2.5 h.The reaction mixture was diluted with EtOAc (50 mL) and the solution was washedwith 1N HCl(aq)(3 x 25 mL), 10% NaHCO3(aq) (25 mL), anddistd H2O (30 mL). Then the organic layer was dried over Na2SO4,filtered and the solvent removed in vacuo. The residue was purified by silicagel chromatography (EtOAc: n-hexane=1:4) to give 2a (926 mg, 84%). IR (KBr) 2973,2360, 1844, 1732, 1683, 1652, 1540, 1456, 1373 cm-1. 1H NMR (500 MHz, CDCl3): δ 5.97 (d, J = 9.7 Hz, 1H), 5.76 (dd, J = 6.1, 9.6 Hz, 1H), 5.50 (s, 1H), 5.36(dd, J = 3.1, 6.1 Hz, 1H), 5.21 (dt, J = 1.7, 3.5 Hz, 1H), 4.45 (m, 1H), 2.73(d, J = 5.3 Hz, 1H), 2.70 (dd, J = 1.5, 3.50 Hz, 1H), 2.46 (m, 1H),2.32 (dd, J = 7.0, 13.8 Hz, 2H), 2.22(m, 1H), 2.06 (s, 3H), 2.03 (m, 2H), 1.90 (dd, J = 2.4, 7.7 Hz, 1H), 1.69 (m, 2H), 1.63 (m, 2H), 1.43 (m, 3H),1.23 (m, 1H), 1.08 (d, J = 6.9 Hz, 3H),1.05 (d, J = 7.4 Hz, 3H), 0.87 (d, J = 7.1 Hz, 3H), 0.83 (t, J = 7.4 Hz, 3H). ESI-MS m/z : 469.3 (M+Na)+.
References:
Lin, Ruo-Kai;Lin, Yuh-Feng;Hsu, Ming-Jen;Hsieh, Chang-Lin;Wang, Chen-Yu;Huang, Chih-Chiang;Huang, Wei-Jan [Bioorganic and Medicinal Chemistry Letters,2016,vol. 26,# 22,p. 5528 - 5533] Location in patent:supporting information