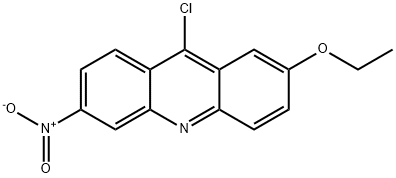
Acridine, 9-chloro-2-ethoxy-6-nitro- synthesis
- Product Name:Acridine, 9-chloro-2-ethoxy-6-nitro-
- CAS Number:20304-69-2
- Molecular formula:C15H11ClN2O3
- Molecular Weight:302.71
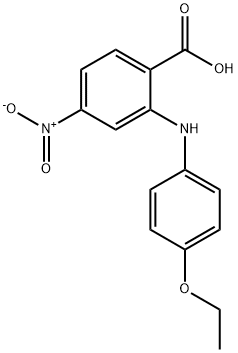
74859-51-1
17 suppliers
$134.00/250mg

20304-69-2
8 suppliers
inquiry
Yield:20304-69-2 88%
Reaction Conditions:
with trichlorophosphateInert atmosphere;Reflux;
Steps:
4.2 4.2.2 Synthesis of 9-chloro-2-ethoxy-6-nitroacridine (24a)
23a (2.00g, 6.62mmol) was added to phosphoryl chloride (15mL) under an Ar atmosphere. The reaction mixture was heated at reflux overnight, then cooled to room temperature, poured into a mixture of aqueous ammonia (100mL), crushed ice (200g), and methylene chloride (200mL), and extracted with methylene chloride. The organic layer was washed with water, dried over sodium sulfate, and concentrated in vacuo. The residue was purified by recrystallization from chloroform to yield 24a (1.76g, 88%) as yellow needles. 1H NMR (500MHz, CDCl3) δ 9.15 (1 H, d, J=2.2Hz), 8.53 (1 H, d. J=9.5Hz), 8.35 (1 H, dd, J=2.2, 9.5Hz), 8.17 (1 H, d, J=9.4Hz), 7.58 (1 H, dd, J=2.6, 9.4Hz), 7.53 (1 H, d, J=2.6Hz), 4.30 (2 H, q, J=7.0Hz), 1.57 (3 H, t, J=7.0Hz).
References:
Ishigami-Yuasa, Mari;Watanabe, Yuko;Mori, Takayasu;Masuno, Hiroyuki;Fujii, Shinya;Kikuchi, Eriko;Uchida, Shinichi;Kagechika, Hiroyuki [Bioorganic and Medicinal Chemistry,2017,vol. 25,# 14,p. 3845 - 3852]
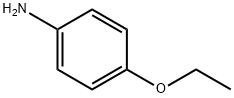
156-43-4
251 suppliers
$5.00/5G

20304-69-2
8 suppliers
inquiry
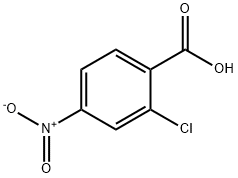
99-60-5
328 suppliers
$19.10/100g

20304-69-2
8 suppliers
inquiry

74859-51-1
17 suppliers
$134.00/250mg

7732-18-5
507 suppliers
$13.50/100ML

20304-69-2
8 suppliers
inquiry