AKOS B005891 synthesis
- Product Name:AKOS B005891
- CAS Number:51336-47-1
- Molecular formula:C11H11BrO4
- Molecular Weight:287.11
Yield:51336-47-1 98%
Reaction Conditions:
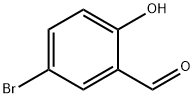
Stage #1: 5-bromosalicyclaldehydewith sodium hydride in N,N-dimethyl-formamide at 0; for 1 h;
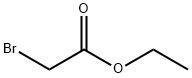
Stage #2: ethyl bromoacetate in N,N-dimethyl-formamide at 0 - 20; for 18 h;
Steps:
1
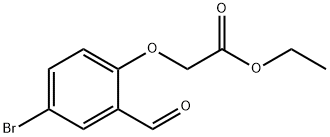
EXAMPLE 1 Preparation of Ethyl(4-bromo-2-formylphenoxy)Acetate; [0079] EMI298.0[0080] This compound was prepared according to a literature procedure (Yoo et al., Bioorg. Med. Chem. 5:445, 1997). 5-Bromo-2-hydroxybenzaldehyde (10.0 g, 49.7 mmol) was dissolved in 90 mL anhyd. DMF and the mixture was cooled to 0[deg.] C. NaH (60% dispersion in mineral oil, 2.38 g, 59.6 mmol) was added in portions and the reaction was stirred at 0[deg.] C. for 1 hour. Ethyl bromoacetate (9.95 g, 59.6 mmol) was then added dropwise over 10 minutes and the reaction was stirred at rt overnight (18 hours). After this time, 30 mL of 1 N HCl was added to adjust the pH to acidity, and the reaction mixture was transferred to a separatory funnel and extracted with EtOAc (3*60 mL). The combined organics rinsed with water (2*40 mL) and brine (40 mL), dried over MgSO4, filtered, and concentrated in vacuo to provide a thick oil. Purification by flash chromatography (10% EtOAc/hexanes) provided the product as a clear oil (14.0 g, 98%). <1>H-NMR (CD2Cl2, [delta]): 1.29 (t, 3H), 4.26 (q, 2H), 4.78 (s, 2H), 6.83 (d, 1H), 7.65 (dd, 1H), 7.94 (s, 1H), 10.47 (s, 1H); LRMS (GC/MS/EI) 286 [M]<+>.
References:
US2003/195352,2003,A1 Location in patent:Page/Page column 31