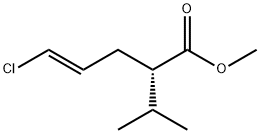
Aliskiren inter-11 synthesis
- Product Name:Aliskiren inter-11
- CAS Number:387353-77-7
- Molecular formula:C9H15ClO2
- Molecular Weight:190.67

67-56-1
790 suppliers
$7.29/5ml-f
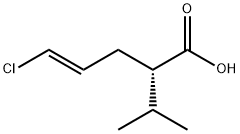
324519-66-6
36 suppliers
$500.31/5MG

387353-77-7
24 suppliers
$364.00/250mg
Yield: 99.7 % ee
Reaction Conditions:
with thionyl chloride for 4 h;Heating / reflux;
Steps:
9
300 ml of sulfuric acid and 300 g (0.802 mol) of N- ( (2S,4E) -5-chloro-2-isopropylpent-4-enoyl) camphorsultam were introduced into a 1 L round bottom flask. The suspension was stirred for 3 hours at 25-300C, then quenched into 600 ml of toluene and 600 ml of water. The aqueous layer was extracted twice with 150 ml of toluene and then the combined organic layers were washed with 300 ml of water. The organic layer was treated with activated charcoal, filtered and then concentrated under vacuum. 1200 ml of heptanes were added to the residue, the
References:
F.I.S. FABBRICA ITALIANA SINTETICI S.p.A WO2008/6394, 2008, A1 Location in patent:Page/Page column 30-31
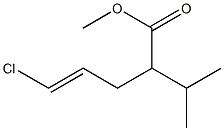
475562-17-5
0 suppliers
inquiry

387353-77-7
24 suppliers
$364.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

67-56-1
790 suppliers
$7.29/5ml-f
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

387353-77-7
24 suppliers
$364.00/250mg

475562-17-5
0 suppliers
inquiry

387353-77-7
24 suppliers
$364.00/250mg
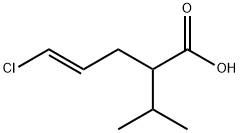
87953-16-0
5 suppliers
inquiry

18107-18-1
210 suppliers
$33.00/5g

387353-77-7
24 suppliers
$364.00/250mg