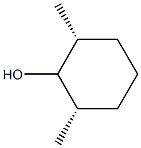
ALPHA,BETA,BETA-2,6-DIMETHYLCYCLOHEXANOL synthesis
- Product Name:ALPHA,BETA,BETA-2,6-DIMETHYLCYCLOHEXANOL
- CAS Number:42846-29-7
- Molecular formula:C8H16O
- Molecular Weight:128.21
Yield:39170-84-8 42% ,42846-29-7 36%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 20; for 1 h;Inert atmosphere;
Steps:
Reduction of cis-2,6-Dimethylcyclohexanone with LithiumAluminum Hydride.
An oven-dried 100-ml three-neck flask, equipped with magneticstirrer, rubber septum, and gas inlet was filled with N2. Tetrahydrofuran(anhydrous, 20 ml) was added followed by a 1 M solution oflithium aluminum hydride in tetrahydrofuran (13.1 ml, 13.1 mmol,1.10 Eq). cis-2,6-Dimethylcyclohexanone (1.46 g, 11.5 mmol) was thenadded dropwise via a syringe and the reaction was stirred at roomtemperature for 1 hour.The reaction was quenched with 2 ml water added dropwise, thendiluted with 5 ml 15% NaOH solution, followed by another 2 ml water.The mixture was filtered through silica gel under a vacuum and rinsedwith diethyl ether (approximately 50 ml). The filtrate was transferredto a 250-ml separatory funnel. The aqueous layer was extracted withethyl ether (3 50 ml), and the combined ether layers were dried overanhydrous MgSO4. 1H NMR on the crude product (1.65 g, 100%)showed a mixture of cis,cis-2,6-dimethylcyclohexanol (54%) and trans,trans-2,6-dimethylcyclohexanol (46%). Flash column chromatographyof the crude product using 240 g silica gel and 10:1 petroleum ether toethyl ether as eluant gave the cis,cis-isomer (646 mg, 42%), a combinationof both isomers (41 mg, 3%), and the trans,trans-isomer(551 mg, 36%). The total yield was 1.24 g (9.65 mmol, 81%).
References:
Chowdhury, Luvana;Croft, Celine J.;Goel, Shikha;Zaman, Naina;Tai, Angela C.-S.;Walch, Erin M.;Smith, Kelly;Page, Alexandra;Shea, Kevin M.;Hall, C. Dennis;Jishkariani;Pillai, Girinath G.;Hall, Adam C. [Journal of Pharmacology and Experimental Therapeutics,2016,vol. 357,# 3,p. 570 - 579]

471256-22-1
0 suppliers
inquiry

42846-29-7
1 suppliers
inquiry
576-26-1
340 suppliers
$10.00/25g

42846-29-7
1 suppliers
inquiry
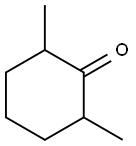
2816-57-1
71 suppliers
$63.20/25g

42846-29-7
1 suppliers
inquiry

2816-57-1
71 suppliers
$63.20/25g

42846-29-7
1 suppliers
inquiry

39170-84-8
1 suppliers
inquiry

42846-29-7
1 suppliers
inquiry