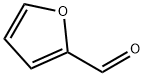
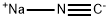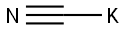α-amino-2-furan acetonitrile synthesis
- Product Name:α-amino-2-furan acetonitrile
- CAS Number:62626-61-3
- Molecular formula:C6H6N2O
- Molecular Weight:122.13
Yield:62626-61-3 64.7 g
Reaction Conditions:
with ammonium acetate in water;ethyl acetate at 15 - 24;
Steps:
1.j Example 1j.4,6-dibromo-3-hydroxypyridinecarbonitrile
a 500 ml flask for one of the stirrer rods,Add 36 grams of ammonium acetate (467 millimolar) to 200 ml of ethyl acetate.And 7.5 grams of NaCN (153 millimoles).The residual sodium cyanide was washed into the flask using 75 ml of water and washed away from the funnel. Then, furfural (12.7 ml, 14.7 g, 153 mmol) was quickly added to the reactor via a syringe. The temperature in the reactor increased from about 15oC to 24oC.The reaction was allowed to stir overnight at ambient temperature (18 ° C). Turn off the agitation to separate the two liquid phases. The organic phase was then sampled for 1 H NMR analysis.And the reaction was judged to be completed only about 80%. then,The reaction was stirred at 25 ° C (using a water bath) for an additional 6 hours.The reaction was shown to be about 90% complete by 1 H NMR.75 ml of 20% aqueous sodium carbonate was added to the reactor and allowed to stir for 30 minutes, thenThe mixture was allowed to stand without stirring for 20-30 minutes. The aqueous phase is removed and thenThe organic phase of the α-amino nitrile containing furfural in ethyl acetate was washed with 2 x 50 ml of saturated brine.
References:
TW2018/27404,2018,A Location in patent:Paragraph 0036; 0037