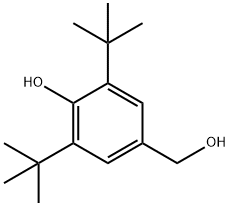
Antioxidant 1330 synthesis
- Product Name:Antioxidant 1330
- CAS Number:1709-70-2
- Molecular formula:C54H78O3
- Molecular Weight:775.2
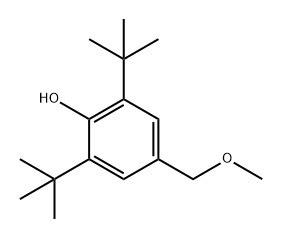
87-97-8
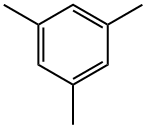
108-67-8

1709-70-2
(1) Add Catalyst B to 1,3,5-trimethylbenzene with uniform stirring to prepare mixture a; (2) Dissolve 3,5-di-tert-butyl-4-hydroxybenzyl methyl ether in dichloromethane with stirring to prepare mixture b; (3) Slowly add mixture b dropwise to mixture a under stirring conditions and react at 10 °C for 2 h. Subsequently, cool to room temperature to obtain mixture c; (4) The mixture c was filtered, the catalyst B was recovered and the filtrate d was collected; (5) The filtrate d was concentrated to dryness to obtain the crude product. The crude product was purified by n-heptane recrystallization: n-heptane was added to the crude product, heated to 85 °C and maintained for 2 hours, subsequently cooled to room temperature for crystallization, filtered to collect the crystals, and dried under vacuum to obtain antioxidant 330 (Compound II).
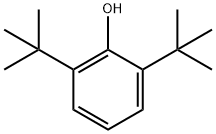
128-39-2
363 suppliers
$5.00/25g
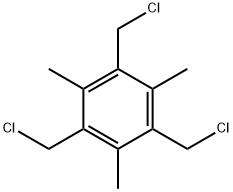
3849-01-2
39 suppliers
$7.00/250mg

1709-70-2
266 suppliers
$7.00/10g
Yield:1709-70-2 99.5%
Reaction Conditions:
with hydrogenchloride;15-crown-5;3,4,7,8-Tetramethyl-o-phenanthrolin;cadmium(II) iodide;ytterbium(III) triflate in tetrachloromethane;water at 50; for 10 h;Catalytic behavior;Concentration;Reagent/catalyst;Temperature;Solvent;
Steps:
1 Example 1
Add 100 mmol of the compound of the formula (I) and 320 mmol of the compound (II) to a suitable amount of a mixture of carbon tetrachloride and 15-crown-5 in a volume ratio of 5:1 at room temperature. Then adding a ternary composite catalyst (a mixture of Yb(OTf)3,3,4,7,8-tetramethyl-1,10-phenanthroline and CdI2, The mass ratio of the three is 1:0.2:0.05, the mass of the compound of the formula (I) and the ternary composite catalyst is 1:0.03), and the aqueous solution of dilute hydrochloric acid having a concentration of 3% by mass (the compound of the formula (I) and the rare The mass ratio of the aqueous hydrochloric acid solution is 1:0.5); Then, the temperature was raised to 50 ° C with stirring, and the reaction was carried out at this temperature for 10 hours. After the completion of the reaction, the mixture was filtered while hot, and the filtrate was washed thoroughly with deionized water 2-3 times. The organic phase was separated, concentrated under reduced pressure, and the residue was recrystallized from petroleum ether to give the desired product antioxidant 330. The yield was 99.5%.
References:
CN104788292,2016,B Location in patent:Paragraph 0065-0066; 0071; 0074; 0077; 0082; 0090; 0095

87-97-8
7 suppliers
inquiry

108-67-8
387 suppliers
$5.00/5G

1709-70-2
266 suppliers
$7.00/10g
![4,4'-[2,4,6-Trimethyl-1,3-phenylenebis(methylene)]bis[2,6-di(tert-butyl)phenol]](/CAS/GIF/41642-52-8.gif)
41642-52-8
1 suppliers
inquiry