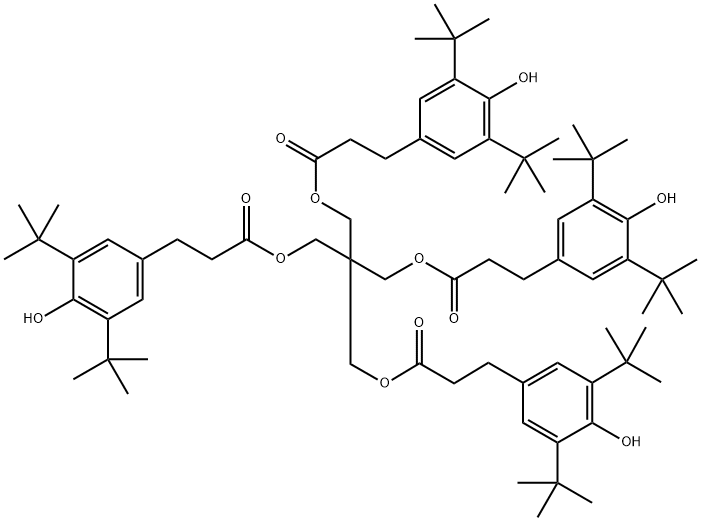
Antioxidant 1010 synthesis
- Product Name:Antioxidant 1010
- CAS Number:6683-19-8
- Molecular formula:C73H108O12
- Molecular Weight:1177.66
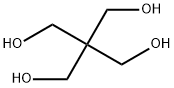
115-77-5
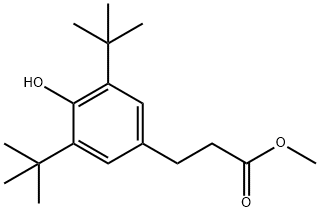
6386-38-5

6683-19-8
Under mechanical stirring, 584 g of methyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate, 68 g of pentaerythritol, 0.58 g of sodium montmorillonite, and 50 mL of methanol were added to a 1000 mL four-neck flask. A thermometer sleeve was installed and stirring was started. The reaction was heated to 130-140 °C via a heating jacket while nitrogen was charged and the pressure of the reaction system was controlled at 3-4 MPa. After the reaction lasted for 7 h, the reaction was cooled down to 25 °C, filtered and dried to obtain pentaerythritol tetrakis[3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate]. The results were analyzed by liquid chromatography as follows: the absorption peak with a retention time of 2.643 min corresponded to methyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate with 0.43%; the absorption peak with a retention time of 3.29 min corresponded to pentaerythritol tris[3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate] with 0.69%; the absorption peak with a retention time of The absorption peak at 3.84 min corresponded to dealkylated pentaerythritol tetrakis[3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate] with 0.55%; the absorption peak with retention time of 4.747 min corresponded to pentaerythritol tetrakis[3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate] with 96.45%. The purity of pentaerythritol tetrakis[3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate] synthesized by this method meets the industrial standard of not less than 94%, and the yield of the product is 98%.

115-77-5
0 suppliers
$10.00/10g

6386-38-5
180 suppliers
$13.00/10g

6683-19-8
494 suppliers
$8.00/10g
Yield:6683-19-8 98%
Reaction Conditions:
with sodium montmorillonite in methanol at 130 - 140; under 22502.3 - 30003 Torr; for 7 h;Inert atmosphere;Reagent/catalyst;
Steps:
1 Example 1
The 584gβ- (3,5- di-t-butyl-4-hydroxyphenyl) propionate,68 g of pentaerythritol,0.58 g of sodium montmorillonite,50 ml of methanol was added to the flask with mechanical stirring,Thermometer socket,In a 1000 ml four-necked flask equipped with a stirrer,Heating the material with a heating jacket,And stirring is started,The temperature reaches 130-140 ,Filled with nitrogen,The reaction system pressure was controlled at 3-4 MPa,7h after the end of the reaction,Cooling to 25 ,filter,drying,Pentaerythritol tetrakis [β - (3,5-di-tert-butyl-4-hydroxyphenyl) propionate]. The results of liquid chromatography, the results shown in Figure 1,The absorption peak at the retention time of 2.643 min belongs to 3.5 methyl ester with the content of 0.43%.The absorption peak at the retention time of 3.29 min is attributed toPentaerythritol tris [β- (3,5-di-tert-butyl-4-hydroxyphenyl) propionate], content of 0.69%;The absorption peak at 3.84 min was assigned to dealkylationPentaerythritol tetrakis [β- (3,5-di-tert-butyl-4-hydroxyphenyl) propionate] in 0.55%;The absorption peak at the retention time of 4.747 min is attributed toPentaerythritol tetrakis [β- (3,5-di-tert-butyl-4-hydroxyphenyl) propionate] in 96.45%Therefore, the synthesis of pentaerythritol tetrakis [[beta] - (3,5-di-tert-butyl-4-hydroxyphenyl) propionate] in the present method meets the industry standard of not less than 94%. The yield of the product was 98%.
References:
CN106278894,2017,A Location in patent:Paragraph 0017; 0018; 0019
