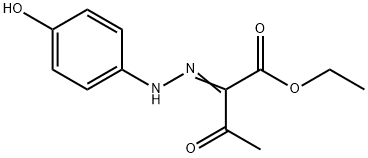
Apixaban Impurity 42 synthesis
- Product Name:Apixaban Impurity 42
- CAS Number:132577-23-2
- Molecular formula:C12H14N2O4
- Molecular Weight:250.25
Yield:132577-23-2 78%
Reaction Conditions:
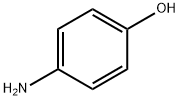
Stage #1: 4-amino-phenolwith hydrogenchloride;sodium nitrite in water at 0 - 5;
Stage #2: ethyl acetoacetatewith sodium acetate in ethanol;water;Cooling with ice;
Steps:
The aromatic amine (1-13) (0.01 mol) was dissolved in a mixture of 4.0 mL of concentrated hydrochloric acid (HCl) and 4.0 mL of distilled water. The amine hydrochloride solution was kept at freezing temperature. To this, an aqueous solution of sodium nitrite (NaNO2) 0.69 g (0.01 mol) in 5.0 mL of distilled water was added dropwise with continuous stirring, keeping the temperature of the reaction vessel at 0-5 °C. Meanwhile in another beaker, 6.8 g (0.05 mol) of sodium acetate (CH3COONa) in a solution of 1.53 mL (0.012 mol) ethyl acetoacetate in 25.0 mL of ethyl alcohol was taken and cooled in an ice-bath. Now the diazotized solution was added to this solution dropwise with thorough stirring. The reaction mixture was kept overnight, filtered under suction, washed thoroughly with cold water, dried and recrystallised from a mixture of dimethylformamide (DMF) and ethyl alcohol (EtOH) (3:7, v/v) to give the corresponding butanoate.
References:
Bandyopadhyay, Prabal;Guha, Lopamudra;Seenivasagan;Sathe, Manisha;Sharma, Pratibha;Parashar;Kaushik [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 2,p. 794 - 797] Location in patent:supporting information; experimental part