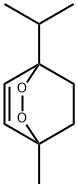
ASCARIDOLE synthesis
- Product Name:ASCARIDOLE
- CAS Number:512-85-6
- Molecular formula:C10H16O2
- Molecular Weight:168.23
Yield:512-85-6 80%
Reaction Conditions:
with dihydrogen peroxide in dichloromethane at 20; for 3 h;Solvent;
Steps:
3.3. General procedure for oxygenation of substrates (3a-g) with the 2b/H2O2 system
General procedure: To a suspension of substrates (3a-g, 0.5 mmol) and 35% hydrogen peroxide (443 μL, 5 mmol) in dichloromethane (4 mL) was added PDAIS (2b) (348 mg, 1 mmol) dropwise within 1 h at room temperature. The mixture was further stirred for 2 h. After removal of the solvent, ether was added to the reaction mixture to precipitate iodopolystyrene (PS-I). The formed PS-I was recovered by washing with ether (2 x 15 mL). After the addition of 4-methoxyacetophenone as an internal standard, the organic phase was washed with water (2 x 10 mL) and brine, dried over Na2SO4 , and concentrated. The conversion of 3a-g, formation of 4a-g, and product distribution were determined directly by 1H NMR analysis of the crude mixture. Then the resulting mixture was chromatographed on a short column (silica gel, 13-15 g) using a mixture of hexane and Et2O (90:10) as an eluent to afford the desired oxygenated products.
References:
?atir, Mustafa [Turkish Journal of Chemistry,2017,vol. 41,# 4,p. 467 - 475]
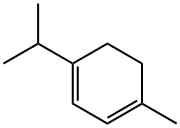
99-86-5
299 suppliers
$13.00/5g

512-85-6
58 suppliers
inquiry
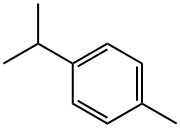
99-87-6
249 suppliers
$14.00/25mL

512-85-6
58 suppliers
inquiry

99-86-5
299 suppliers
$13.00/5g
![3,8-Dioxatricyclo[5.1.0.02,4]octane, 4-methyl-7-(1-methylethyl)-](/CAS/20210305/GIF/104975-22-6.gif)
104975-22-6
0 suppliers
inquiry

512-85-6
58 suppliers
inquiry

99-87-6
249 suppliers
$14.00/25mL