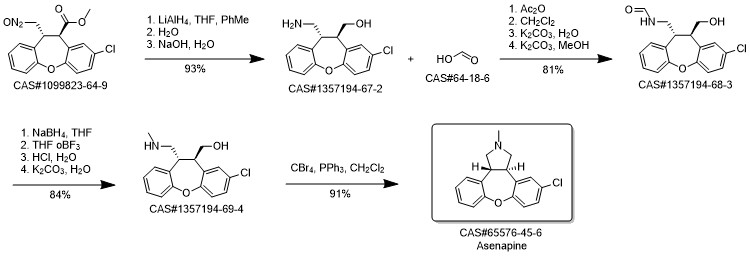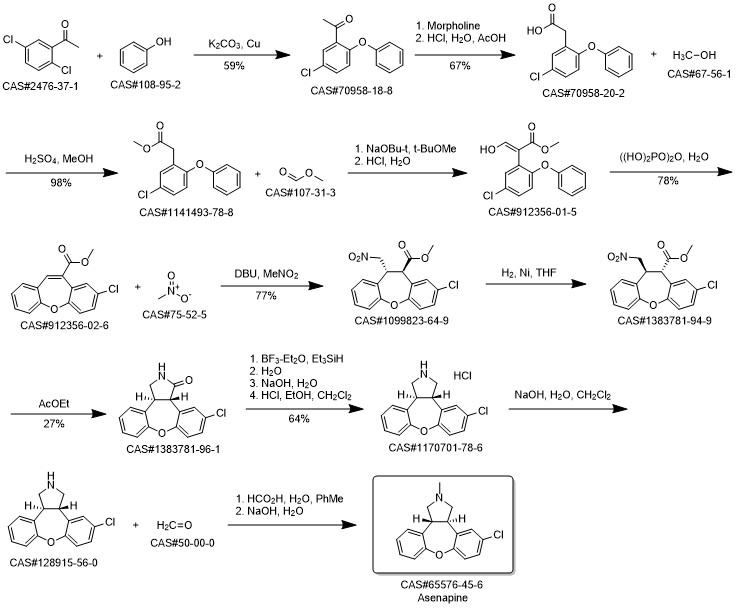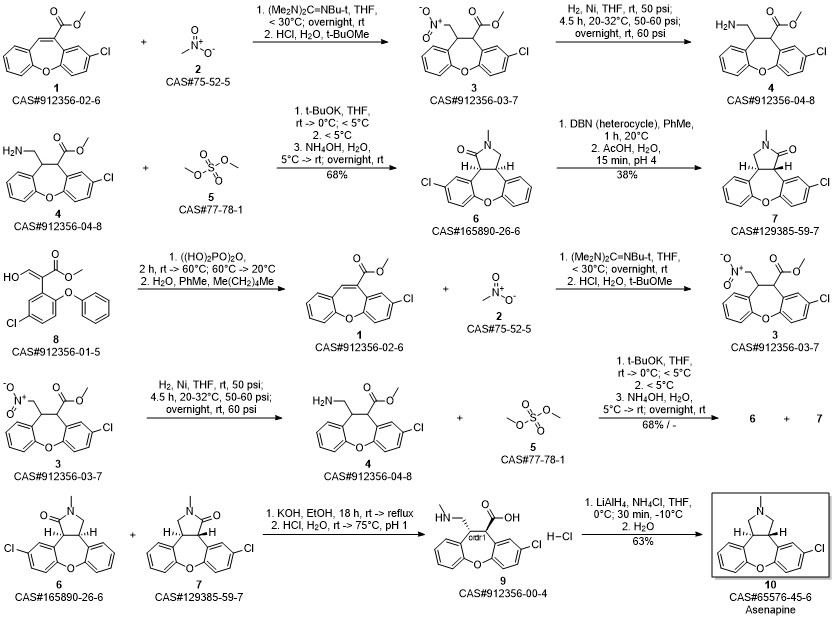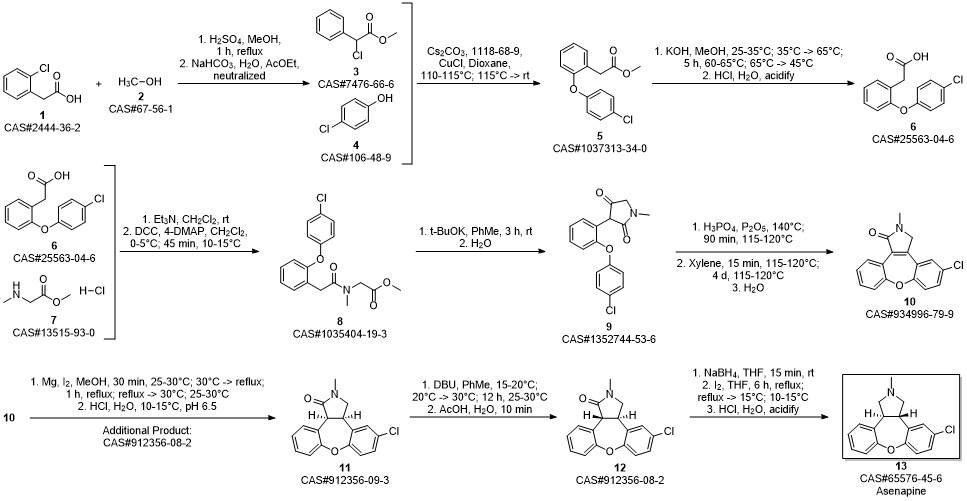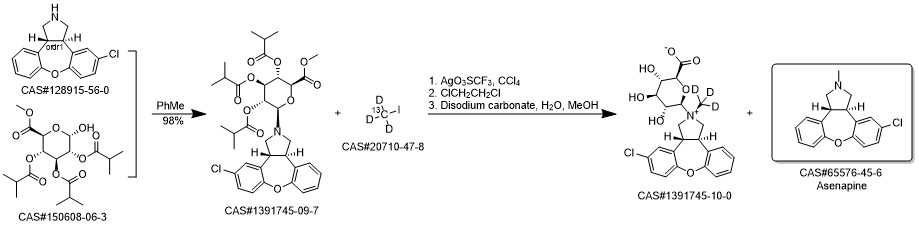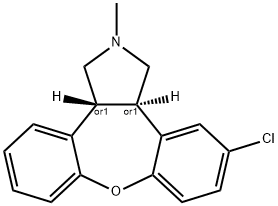
Asenapine synthesis
- Product Name:Asenapine
- CAS Number:65576-45-6
- Molecular formula:C17H16ClNO
- Molecular Weight:285.772
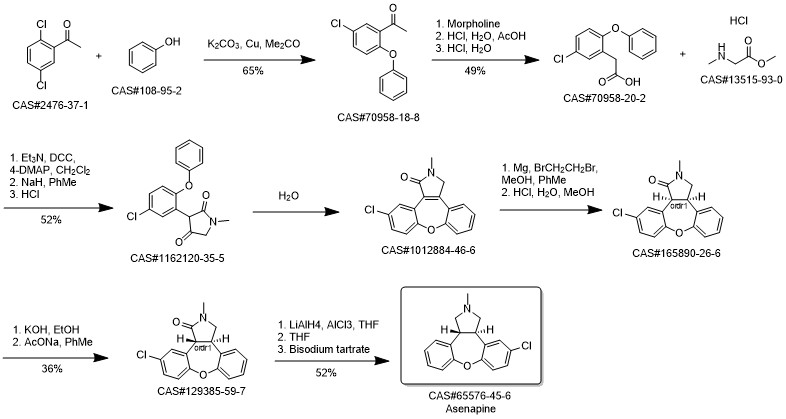
Synthesis of asenapine; Zhang, Xiao-ying; Zheng, Guo-jun Huaxue Shiji Volume 33 Issue 12 Pages 1135-1137 Journal 2011
![trans-(+/-)-11-Chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenz[2,3:6,7]oxepino[4,5-c]pyrrol-1-one](/CAS/20180906/GIF/129385-59-7.gif)
129385-59-7
150 suppliers
$12.00/1g

65576-45-6
146 suppliers
$45.00/1mg
Yield:65576-45-6 100%
Reaction Conditions:
with aluminum (III) chloride;lithium aluminium tetrahydride in tetrahydrofuran at -25 - -20; for 1 h;Solvent;
Steps:
2.a a) Preparation of trans-11-chloro-2,3,3a,12b-tetrahydro-2-methyl-lH-dibenzo[2,3:6,7] oxepino[4,5-c]pyrrole (Asenapine base):
a) Preparation of trans-ll-chloro-2,3,3a,12b-tetrahydro-2-methyl-lH-dibenzo[2,3:6,7] oxepino[4,5-c]pyrrole (Asenapine base): Aluminum chloride (13.87 gm) was added in portions to tetrahydrofuran (600 ml) at -20 to - 25°C. Under stirring lithium aluminum hydride (14.92 gm) was added in portions to the reaction mixture while keeping the temperature between -20° C to -25° C. The mixture was stirred for 20 minutes. Trans-l l-chloro-2,3,3a,12b-tetrahydro-2-methyl-lH-dibenz[2,3:6,7] oxepino [4,5-c]pyrrol-l-one (40.0 gm) was dissolved in tetrahydrofuran (400 ml) and was then slowly added to the reaction mixture while keeping the temperature between -20° C to - 25° C. Stirring was continued for 1 hour at temperature between -20° C to -25° C. 0.6 N sodium hydroxide solution (400 ml) was slowly added without controlling the temperature. The reaction mass was diluted with toluene (800 ml) and water (800 ml) and stirred for 30 minutes and the reaction mixture was filtered. The filtrate was separated. The aqueous layer was extracted twice with toluene (2 X 800 ml). The toluene layers were combined and washed with water (800 ml). The toluene layer was distilled out under reduce pressure at 50° C to obtain (38.13 gm) of trans-l l-chloro-2,3,3a,12b-tetrahydro-2-methyl-lH-dibenz [2,3:6,7] oxepino [4,5-c]pyrrole (Asenapihe base). Total yield: 100.0% Impurity VII: 0.14%; Impurity VIII: not detected; Impurity X : not detected; Impurity IXa and IXb : 0.1%; Impurity *M' RRT-3.1: 0.43% HPLC Purity: 98.59
References:
MEGAFINE PHARMA (P) LTD.;MATHAD, Vijayavitthal Thippannachar;SOLANKI, Pavankumar Vrajlal;UPPELLI, Sekhar Babu;CHAVAN, Shravan Ratan WO2013/24492, 2013, A2 Location in patent:Page/Page column 22; 23
85650-56-2
255 suppliers
$15.00/10mg

65576-45-6
146 suppliers
$45.00/1mg
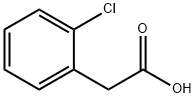
2444-36-2
452 suppliers
$5.00/25g

65576-45-6
146 suppliers
$45.00/1mg