Asenapine
- CAS No.
- 65576-45-6
- Chemical Name:
- Asenapine
- Synonyms
- Saphris;HSDB8061;Asenapine;HSDB 8061;HSDB-8061;Unii-jkz19V908o;Einecs 265-829-4;Asenapine [inn:ban];Asenapine USP/EP/BP;Asenapine free base
- CBNumber:
- CB5879263
- Molecular Formula:
- C17H16ClNO
- Molecular Weight:
- 285.772
- MDL Number:
- MFCD09838016
- MOL File:
- 65576-45-6.mol
- MSDS File:
- SDS
| Boiling point | 357.9±42.0 °C(Predicted) |
|---|---|
| Density | 1.231 |
| storage temp. | Store at +4°C |
| solubility | DMSO:50.0(Max Conc. mg/mL);174.97(Max Conc. mM) |
| form | A solid |
| pka | 9.50±0.20(Predicted) |
| color | White to off-white |
| FDA UNII | JKZ19V908O |
| ATC code | N05AH05 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319-H315-H335 | |||||||||
| Precautionary statements | P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P | |||||||||
| NFPA 704 |
|
Asenapine price More Price(21)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 9000496 | (±)-Asenapine ≥95% | 65576-45-6 | 1mg | $44 | 2024-03-01 | Buy |
| Cayman Chemical | 9000496 | (±)-Asenapine ≥95% | 65576-45-6 | 5mg | $93 | 2024-03-01 | Buy |
| Cayman Chemical | 9000496 | (±)-Asenapine ≥95% | 65576-45-6 | 10mg | $153 | 2024-03-01 | Buy |
| TRC | A788000 | Asenapine | 65576-45-6 | 25mg | $435 | 2021-12-16 | Buy |
| ChemScene | CS-0860 | Asenapine 98.81% | 65576-45-6 | 10mg | $144 | 2021-12-16 | Buy |
Asenapine Chemical Properties,Uses,Production
Description
(±)-Asenapine is an atypical antipsychotic. It binds to dopamine D1-4, α-adrenergic, and histamine receptors (Kis = 0.42-1.45, 0.32-1.26, and 1-6.17 nM, respectively), as well as the serotonin (5-HT) receptor subtypes 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT5A, 5-HT6, and 5-HT7 (Kis = 0.03-3.98 nM). (±)-Asenapine inhibits the suppression of neuron firing induced by the 5-HT2A, dopamine D2, and α2-adrenergic receptor agonists 2,5-dimethoxy-4-iodoamphetamine (DOI), apomorphine, and clonidine , respectively, in rat brain (ED50s = 75, 40, and 85 μg/kg, respectively). In vivo, (±)-asenapine (0.05-0.2 mg/kg, s.c.) increases extracellular dopamine levels in the medial prefrontal cortex (mPFC), nucleus accumbens (NAc), and lateral striatum and suppresses the conditioned avoidance response in rats. It prevents acute and chronic phencyclidine-induced deficits in cued reversal learning in rats when administered at a dose of 0.075 mg/kg. Formulations containing asenapine have been used in the treatment of schizophrenia and bipolar I disorder.
Chemical Properties
Yellow Oil
Uses
Combined serotonin (5HT2) and dopamine (D2) receptor antagonist; structurally related to Mianserin. Antipsychotic
Definition
ChEBI: (R,R)-asenapine is a 5-chloro-2-methyl-2,3,3a,12b-tetrahydrodibenzo[2,3:6,7]oxepino[4,5-c]pyrrole in which both of the stereocentres have R configuration. It is a conjugate base of a (R,R)-asenapine(1+). It is an enantiomer of a (S,S)-asenapine.
Biological Activity
Novel psychopharmacologic agent. Displays antagonist activity at 5-HT, dopamine, noradrenalin and histamine receptor subtypes (pK i values are 8.60, 8.40, 10.15, 9.75, 10.46, 8.84, 9.60, 9.94, 8.85, 8.90, 8.84, 9.38, 8.95, 8.93, 8.9, 9.49, 8.91, 9.00 and 8.21 for 5-HT 1A , 5-HT 1B , 5-HT 2A , 5-HT 2B , 5-HT 2C , 5-HT 5A , 5-HT 6 , 5-HT 7 , D 1 , D 2L , D 2S , D 3 , D 4 , α 1A , α 2A , α 2B , α 2C , H 1 and H 2 receptors respectively). Displays no appreciable affinity for muscarinic receptors. Exhibits potent activity in animal models predictive of antipsychotic efficacy.
Clinical Use
Atypical antipsychotic
Treatment of schizophrenia and bipolar disease
Enzyme inhibitor
This atypical antipsychotic agent (FW = 285.77 g/mol; CAS 65576-45-6), marketed under the trade names Saphris ?, and also known as Org 5222 and (3aRS,12bRS)-rel-5-chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenz[2,3: 6,7]-oxepino-[4,5-c]pyrrole, is multi-receptor antagonist with the following spectrum of binding interactions: serotonin 5-HT1A receptor, Ki = 2.5 nM; serotonin 5-HT1B receptor, Ki = 4.0 nM; serotonin 5-HT2A receptor, Ki = 0.06 nM; serotonin 5-HT2B receptor, Ki = 0.16 nM; serotonin 5-HT2C receptor, Ki = 0.03 nM; serotonin 5-HT5A receptor, Ki = 1.6 nM; serotonin 5-HT6 receptor, Ki = 1.5 nM; serotonin 5-HT7 receptor, Ki = 0.13 nM; a1- Adrenergic receptor, Ki = 1.2 nM; a2A-Adrenergic receptor, Ki = 1.2 nM; a2B-Adrenergic receptor, Ki = 0.25 nM; a2C-Adrenergic receptor, Ki = 1.2 nM; dopamine D1-receptor, Ki = 1.4 nM; dopamine D2-receptor, Ki = 1.3 nM; dopamine D3-receptor, Ki = 0.4 nM; dopamine D4-receptor, Ki = 1.1 nM; histamine H1-receptor, Ki = 1.0 nM; and histamine H2-receptor, Ki = 6 nM. Like other atypical antipsychotic drugs, asenapine preferentially enhances dopamine and acetylcholine efflux in the rat medial prefrontal cortex and hippocampus. See Reference-x for asenapine’s UV, IR, NMR, and mass spectra as well as X-ray analysis, thermal properties, solubilities and partition coefficient.
Drug interactions
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Analgesics: increased risk of convulsions with
tramadol; enhanced hypotensive and sedative effects
with opioids.
Anti-arrhythmics: increased risk of ventricular
arrhythmias with anti-arrhythmics that prolong the
QT interval; avoid with amiodarone, disopyramide
and procainamide (risk of ventricular arrhythmias).
Antidepressants: concentration possibly increased
by fluvoxamine; possibly increased paroxetine
concentration; concentration of tricyclics possibly
increased.
Antiepileptics: antagonises anticonvulsant effect.
Antimalarials: avoid with artemether/lumefantrine.
Antivirals: concentration possibly increased by
ritonavir.
Anxiolytics and hypnotics: increased sedative effects.
Metabolism
Metabolism is by direct glucuronidation by UGT1A4
and oxidative metabolism by cytochrome P450
isoenzymes (predominantly CYP1A2) are the primary
metabolic pathways for asenapine.
Excretion is 50% renal and 50% via the faeces.
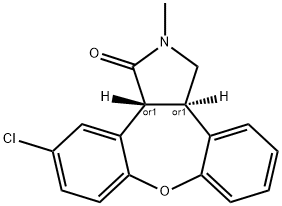
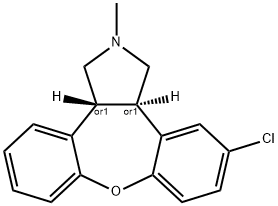
Asenapine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32836 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29888 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49392 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 23899 | 58 |
| Baoji Guokang Bio-Technology Co., Ltd. | 0917-3909592 13892490616 | gksales1@gk-bio.com | China | 9316 | 58 |
| ChemExpress | +86-021-58950125 +86-021-58950125; | info@chemexpress.com | China | 598 | 58 |
| Wuhan Golt Biotech Co., Ltd. | +8615389281203 | maria@goltbiotech.com | China | 980 | 58 |
View Lastest Price from Asenapine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-07-27 | Asenapine USP/EP/BP
65576-45-6
|
US $1.10 / g | 1g | 99.9% | 100 Tons min | Dideu Industries Group Limited | |
 |
2021-07-13 | Asenapine
65576-45-6
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2021-07-09 | Asenapine
65576-45-6
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- Asenapine USP/EP/BP
65576-45-6
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited
-

- Asenapine
65576-45-6
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
-

- Asenapine
65576-45-6
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
65576-45-6(Asenapine)Related Search:
1of4





