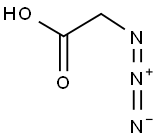
Azidoacetic acid synthesis
- Product Name:Azidoacetic acid
- CAS Number:18523-48-3
- Molecular formula:C2H3N3O2
- Molecular Weight:101.06
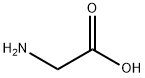
56-40-6
1340 suppliers
$5.00/25g

18523-48-3
76 suppliers
$54.00/1g
Yield:18523-48-3 90%
Reaction Conditions:
with triflic azide;potassium carbonate;copper(II) sulfate in methanol;dichloromethane;water at 20;
Steps:
3.2 3.2 Gly-azide NI1594 [N3-Gly-OH]
A solution of NaN3 (455 mg, 7 mmol) in 1 .2 ml water was added in ice bath and treated with 2 ml of CH2CI2. The resulting biphasic mixture was stirred vigorously and treated with Tf20 (192 μΙ, 1 .4 mmol) over period of 5 min. The reaction mixture was stirred in a ice bath for 2h, then the organic phase was separated and the aqueous phase was extracted with CH2CI2 (2 x 2 ml). The combined organic phase were washed with saturated NaHC03 (3 ml) and use immediately without further purification. Commercial compound Glycine, ( 54 mg, 0.7 mmol) was dissolved in 2.5 ml of water and treated with potassium carbonate (K2C03, 145 mg, 1 .01 mmol) and CuS04 hydrate (1.7 mg 7 μηηοΙ in 0.4 ml water) were added to the flask consecutively. Freshly prepared dichloromethane solution of TfN3 was then added, and the solution was brought to homogeneity by adding MeOH (ca.1.3 ml). The resulting solution was stirred at room temperature overnight. The only organic phase (MeOH/ CH2CI2) was evaporated under vacuum and the remaining aqueous solution was poured into 6 ml of water and the pH value was adjusted to 6 with 0.1 N HCI solution and added to 8 ml of phosphate buffer (0.02M pH=6.2). The acid aqueous phase was washed with AcOEt (4 x 5 ml) and then concentrated to 1/2 of initial volume, acidified around pH =2 with 0.1 N HCI solution and then extracted with AcOEt (5 x 10 ml). The combined organic phase were washed with brine (20 ml), dried over anhydrous Na2S04, filtered and concentrated to give white liquid (64 mg, yield 90 %). The final product was used without further purification and it structure confirmed by 1H-NMR analysis (300 MHz): δ (ppm, CDCI3), 3.97( 2H, s, -CH2-) and Mass spectroscopy ESI-Tof (m/z) [M+H+]= 102.12
References:
LEADIANT BIOSCIENCES SA IN LIQUIDAZIONE;GIANNINI, Giuseppe;NAGGI, Annamaria;ESPOSITO, Emiliano WO2018/188990, 2018, A1 Location in patent:Page/Page column 41
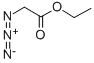
637-81-0
180 suppliers
$40.00/500mg

18523-48-3
76 suppliers
$54.00/1g
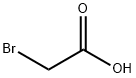
79-08-3
317 suppliers
$9.00/1g

18523-48-3
76 suppliers
$54.00/1g
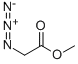
1816-92-8
83 suppliers
$15.00/1g

18523-48-3
76 suppliers
$54.00/1g
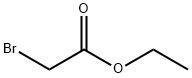
105-36-2
349 suppliers
$5.00/5 g

18523-48-3
76 suppliers
$54.00/1g