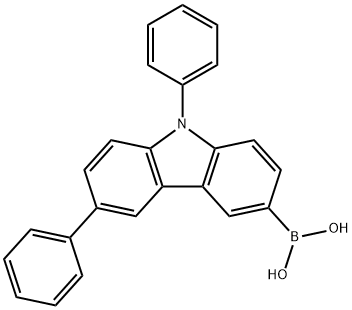
B-(6,9-Diphenyl-9H-carbazol-3-yl)boronic acid synthesis
- Product Name:B-(6,9-Diphenyl-9H-carbazol-3-yl)boronic acid
- CAS Number:1133058-06-6
- Molecular formula:C24H18BNO2
- Molecular Weight:363.22
5419-55-6
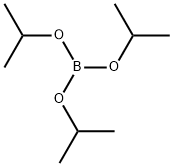
1160294-85-8

1133058-06-6
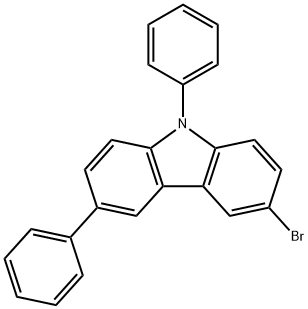
3-Bromo-6,9-diphenyl-9H-carbazole (4.8 g, 12.06 mmol) was dissolved in tetrahydrofuran (60 mL) and cooled to -78 °C. Under nitrogen protection, 2.5 M hexane solution of n-butyllithium (6.3 mL, 15.68 mmol) was slowly added dropwise, and stirring was continued at -78 °C for 1 h after completion of the drop. Subsequently, triisopropyl borate (4.5 g, 24.12 mmol) was slowly added and the reaction mixture was gradually warmed to room temperature and stirring was continued for 12 hours. Upon completion of the reaction, the reaction was quenched by slow dropwise addition of purified water (20 mL). The organic layer was separated by extraction with a mixture of dichloromethane and saturated aqueous ammonium chloride. The organic layer was concentrated and purified by silica gel column chromatography, and finally crystallized with a mixed solvent of dichloromethane/hexane to afford 6,9-diphenyl-9H-carbazol-3-yl-3-boronic acid (3 g, 70% yield).

5419-55-6
386 suppliers
$12.00/5g

1160294-85-8
122 suppliers
$21.00/1g

1133058-06-6
102 suppliers
$45.00/2.5mg
Yield: 70%
Reaction Conditions:
Stage #1:3-bromo-N-phenyl-6-phenylcarbazole with n-butyllithium in tetrahydrofuran;hexane at -78; for 1 h;
Stage #2:Triisopropyl borate in tetrahydrofuran;hexane for 12 h;
Stage #3: with hydrogenchloride in tetrahydrofuran;hexane;water
Steps:
After dissolving compound C-11-2 (4.8 g, 12.06 mmol) in THF (60 mL), 2.5 M n-BuLi in hexane (6.3 mL, 15.68 mmol) was added thereto at -78°C. The reaction mixture was stirred for 1 hour. B(Oi-Pr)3 (4.5 g, 24.12 mmol) was added slowly to the mixture, and then the mixture was stirred for 12 hours. After completing the reaction, purified water 20 mL was slowly dropped stepwise to the mixture. Thereafter, the mixture was extracted with MC/NH4Cl aq. The obtained organic layer was concentrated, was filtered through silica, and then was crystallized with MC/hexane to obtain compound C-11-3 (3 g, 70%).
References:
ROHM AND HAAS ELECTRONIC MATERIALS KOREA LTD.;AHN, Hee-choon;YOON, Seok-keun;MOON, Doo-hyeon;KIM, Hee-sook;LEE, Su-hyun;SHIN, Hyo-nim;LEE, Kyung-joo;PARK, Kyoung-jin;KIM, Nam-kyun;CHO, Young-jun;KWON, Hyuck-joo;KIM, Bong-ok WO2012/121561, 2012, A1 Location in patent:Page/Page column 27
150-46-9
202 suppliers
$12.00/25mL

1160294-85-8
122 suppliers
$21.00/1g

1133058-06-6
102 suppliers
$45.00/2.5mg
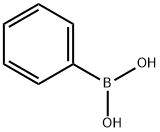
98-80-6
736 suppliers
$5.00/5g

1133058-06-6
102 suppliers
$45.00/2.5mg
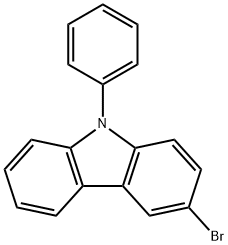
1153-85-1
322 suppliers
$7.00/1g

1133058-06-6
102 suppliers
$45.00/2.5mg
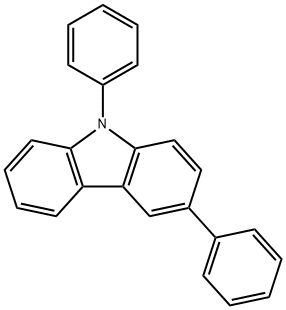
1028648-09-0
6 suppliers
inquiry

1133058-06-6
102 suppliers
$45.00/2.5mg