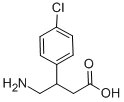
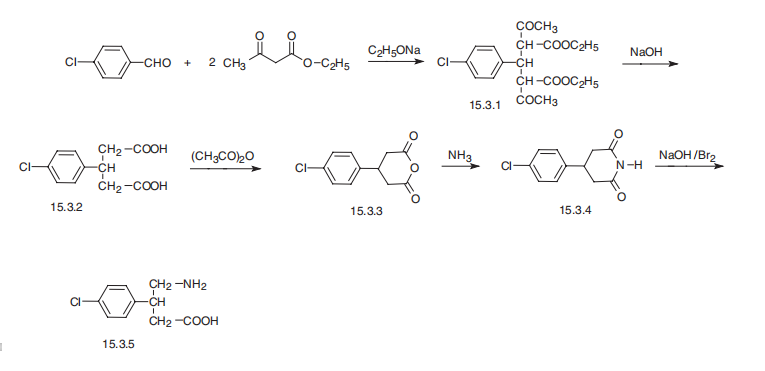
Baclofen synthesis
- Product Name:Baclofen
- CAS Number:1134-47-0
- Molecular formula:C10H12ClNO2
- Molecular Weight:213.66
1141-23-7
159 suppliers
$6.00/1g

1134-47-0
533 suppliers
$11.00/1g
Yield:1134-47-0 79.4%
Reaction Conditions:
with sodium sulfamate;sodium hypobromide at -5 - 55; for 2.16667 h;
Steps:
1e Preparation of baclofen
Intermediate 0.105 g (0.455 mol), 3.6 g (0.03 mol) of sodium sulfamate and freshly prepared sodium hypobromite solution60ml (9% ~ 12%),Stirring at -5 ~ 0 for 1.5 h, slowly warming to 20 ~ 30 ° C after stirring 20min and then heated to 50 ~ 55 ° C again, keep the temperature for 20min, thin layer identification reaction end point (developing agent: n-butanol - glacial acetic acid - water (4: 1: 1),After completion of the reaction, the mixture was cooled to room temperature, the pH was adjusted to 6 to 7 with concentrated hydrochloric acid, allowed to stand for 5 hours,The crude product was recrystallized from isopropanol-water (1: 4) and vacuum dried at 55-60 ° C for 6 h to give 77.2 g of baclofen, a white medicinal complex with a clinical yield of 79.4%
References:
CN106187794,2016,A Location in patent:Paragraph 0026
22518-27-0
131 suppliers
$110.00/1mg

1134-47-0
533 suppliers
$11.00/1g
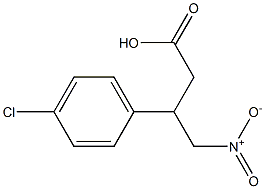
159112-23-9
14 suppliers
inquiry

1134-47-0
533 suppliers
$11.00/1g
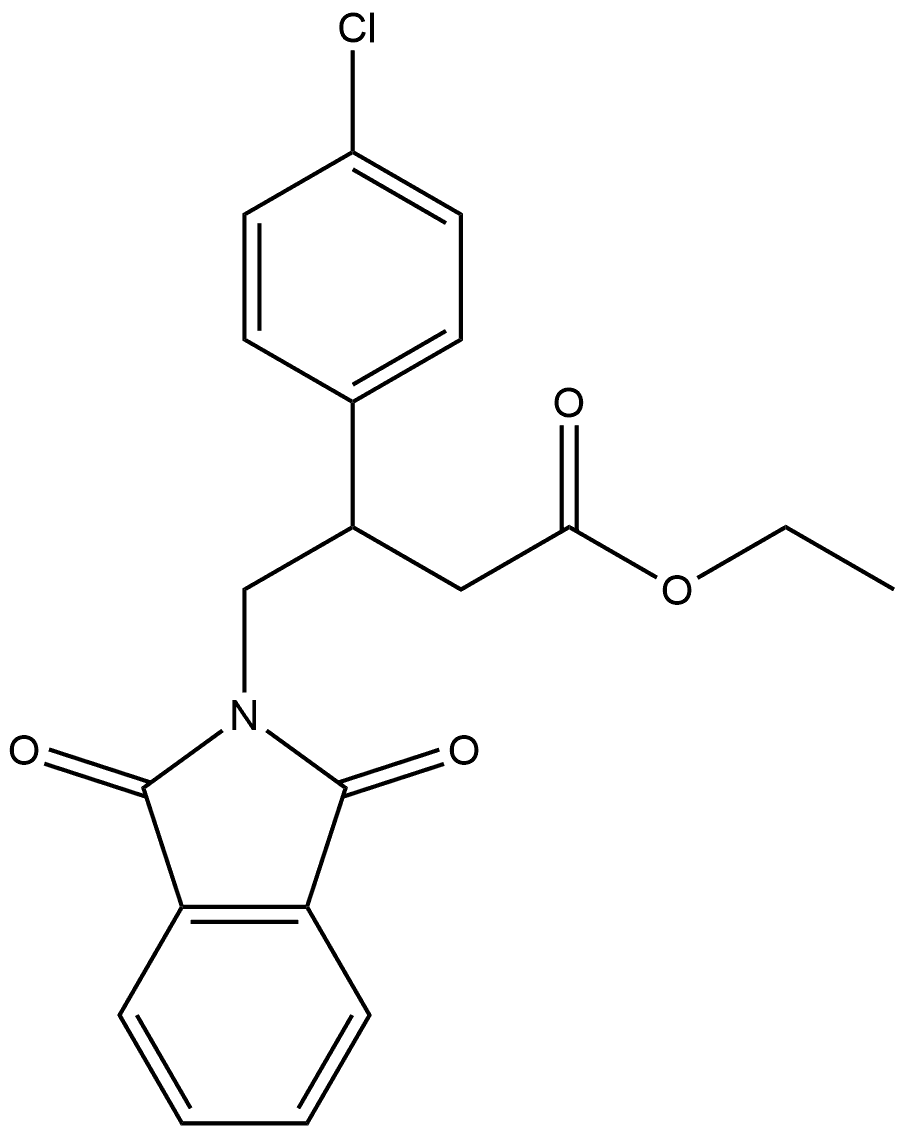
927207-26-9
0 suppliers
inquiry

1134-47-0
533 suppliers
$11.00/1g
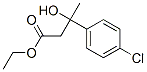
21133-98-2
9 suppliers
inquiry

1134-47-0
533 suppliers
$11.00/1g