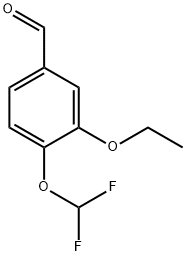
Benzaldehyde, 4-(difluoromethoxy)-3-ethoxy- (9CI) synthesis
- Product Name:Benzaldehyde, 4-(difluoromethoxy)-3-ethoxy- (9CI)
- CAS Number:162401-73-2
- Molecular formula:C10H10F2O3
- Molecular Weight:216.18
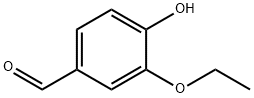
121-32-4
724 suppliers
$5.00/10g
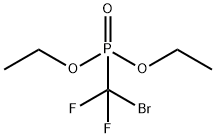
65094-22-6
219 suppliers
$6.00/1g

162401-73-2
16 suppliers
$45.00/50mg
Yield:162401-73-2 70%
Reaction Conditions:
with potassium hydroxide in water;acetonitrile at -78 - 20;Sealed tube;Concentration;
Steps:
4-(difluoromethoxy)-ethoxybenzaldehyde 2: Addition of diethyl(bromodifluoromethyl) phosphonate to aldehyde 1 (Scheme 1).
4-(difluoromethoxy)-ethoxybenzaldehyde 2: Addition of diethyl(bromodifluoromethyl) phosphonate to aldehyde 1 (Scheme 1). Compound 1 (1.00 g,6.02 mmol) was dissolved in CH3CN (30 mL). Solution of 1 was stirred and cooled to -78°c in a bath of dry ice and acetone. KOH (6.75 g, 120.4 mmol) was dissolved indeionized H20 (30 mL) and dripped into the solution of 1. The flask was then sealed with a septum. Diethyl (bromodifluoromethyl) phosphonate (3.21 g, 12.04 mmol) was dripped into the sealed flask and reaction mixture was allowed to warm to rt. The reaction mixture was then left to stir overnight. The work up involved extraction with ether followed by drying the organic layer over Na2504 and gravity filtration into a round bottom flask.Solvent was removed under reduced pressure and compound 2 remained as a brown oil(910 mg, 70%). 1H NMR (400 MHz): 9.93 (d, J = 3.1 Hz, 1H), 7.48 (d, J = 1.8 Hz, 1H),7.45 (dd, J = 8.2, 1.8 Hz, 1H), 7.31 (d, J = 8.0 Hz, 1H), 6.69 (t, J = 74.5 Hz, 1H), 4.18 (q, J= 7.0 Hz, 2H), 1.48 (t, J = 7.0 Hz, 3H). See Figure 1.
References:
WO2015/142670,2015,A1 Location in patent:Page/Page column 16

121-32-4
724 suppliers
$5.00/10g
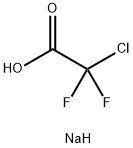
1895-39-2
292 suppliers
$6.00/5g

162401-73-2
16 suppliers
$45.00/50mg