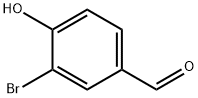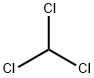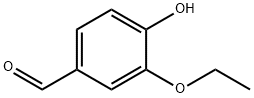
Ethyl vanillin synthesis
- Product Name:Ethyl vanillin
- CAS Number:121-32-4
- Molecular formula:C9H10O3
- Molecular Weight:166.17
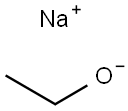
141-52-6
499 suppliers
$10.00/5g
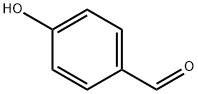
123-08-0
951 suppliers
$5.00/10g

121-32-4
739 suppliers
$5.00/10g
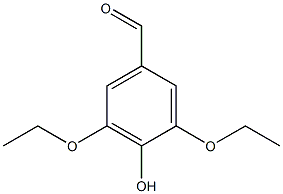
79459-15-7
2 suppliers
inquiry
Yield:121-32-4 91% ,79459-15-7 5.3%
Reaction Conditions:
Stage #1: 4-hydroxy-benzaldehydewith bromine in methanol;chloroform at 0 - 5;
Stage #2: sodium ethanolatewith copper(II) carbonate in ethanol;N,N-dimethyl-formamide at 110 - 120; under 3750.38 - 4500.45 Torr; for 3 h;Inert atmosphere;
Steps:
2
Bromination of p-hydroxybenzaldehydeIn the reactor, 24.4 g (0.2 mol) of p-hydroxybenzaldehyde, 160 ml of methanol, and 250 ml of chloroform were sequentially added, and the mixture was stirred and cooled to 0-5 ° C in an ice water bath. Take 36.8 g of bromine (0.23 mol), dissolve it in 75 ml of chloroform to make a solution, and slowly add it to the reactor dropwise for about 1.5-2 hours, and continue to react for 2.5 hours after the drop. After completion of the reaction, nitrogen bromide and bromine remaining in the system were purged with nitrogen to obtain a reaction completion liquid.The reaction completion liquid is heated, and chloroform and a part of methanol are distilled off to obtain a brominated product, which is directly used as a raw material for vanillin synthesis. The composition and mass fraction of the brominated product was determined to be 93.2% of 3-bromo-p-hydroxybenzaldehyde, and 6.3% of 3,5-dibromo-p-hydroxybenzaldehyde.During the dropwise addition, the hydrogen bromide gas is removed with a slight negative pressure, and it enters the absorption oxidation and chloroform extraction device to make a bromine chloroform solution for recycling.2. Synthesis of methyl vanillinAdd 0.64 moles of sodium methoxide (28% methanol solution) and 80 ml of methanol to the aforementioned reactor of brominated products (0.2 moles), stir well, and then add 1.5 g of copper oxide and dimethylformamide (DMF) in this order. 10 ml. After replacing the air with nitrogen, the temperature was raised to 115 ° C-120 ° C (pressure about 0.5 MPa-0.6 MPa), and the reaction was carried out for 4 hours. The reaction was terminated by cooling to room temperature. The reaction mixture was distilled off methanol. Add 100 ml of water, add 1 g of powdered activated carbon, heat up to 95 ° C with stirring, decolorize for half an hour, filter while hot, cool the filtrate to 60 ° C, neutralize with concentrated hydrochloric acid to pH = 3.6, and precipitate.Continue to cool to 10 ° C, filter, wash and dry the filter cake to obtain a mixture of vanillin and syringaldehyde.3. Pure vanillin and syringaldehyde can be obtained by refining and separating using techniques known in the art; The solvent methanol in Example 1 was replaced by ethanol, and a brominated product (0.2 mol) was charged with 0.7 mol of sodium ethoxide in ethanol, 70 ml of ethanol was stirred and dissolved, and 2.2 g of basic copper carbonate and dimethylformaldehyde were added 12 g of amide (DMF), the air was replaced with nitrogen, and the temperature was raised to 110 ° C-120 ° C (pressure about 0.5MPa-0.6MPa). After 4 hours of reaction, the reaction was cooled to room temperature to terminate the reaction. The reaction mixture was distilled off ethanol and recovered for reuse. Add 350 ml of water, 2 g of powdered activated carbon, heat up to 95 ° C with stirring, decolorize with heat preservation, and filter while hot. The filtrate is cooled to 60 ° C, neutralized with concentrated hydrochloric acid to pH = 2-3, precipitated, and cooled to 10 ° C Filtering, washing and drying to obtain a mixture of ethyl vanillin and 3.5-diethoxy-4-hydroxybenzaldehyde;The yields of ethyl vanillin and 3.5-diethoxy-4-hydroxybenzaldehyde were 91% and 5.3%, respectively.
References:
CN110606801,2019,A Location in patent:Paragraph 0023-0031; 0032; 0033; 0034
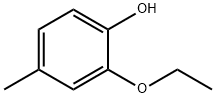
2563-07-7
66 suppliers
$28.00/1G

121-32-4
739 suppliers
$5.00/10g
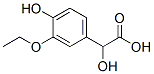
39549-22-9
6 suppliers
inquiry

121-32-4
739 suppliers
$5.00/10g