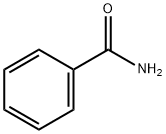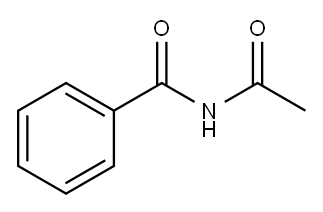
Benzamide, N-acetyl- (6CI,7CI,8CI,9CI) synthesis
- Product Name:Benzamide, N-acetyl- (6CI,7CI,8CI,9CI)
- CAS Number:1575-95-7
- Molecular formula:C9H9NO2
- Molecular Weight:163.17
Yield:1575-95-7 97%
Reaction Conditions:
with sulfuric acid at 100; for 0.05 h;Microwave irradiation;
Steps:
4.2. Typical procedure for the synthesis of N-acylation of amide derivatives
General procedure: Amide derivatives (1.0 mmol), acid anhydrides (3.0 mmol), and 2 drops of concd H2SO4 were placed in a 50 mL round bottom flask equipped with a reflux condenser. The reaction flask was microwave irradiated (50 W, 100 °C) for 3 min with stirring. After the reaction, the solution was extracted with ethyl acetate (2×10 mL), and the combined organic layer was washed with 5% aq NaHCO3 (2×10 mL), and brine (20 mL), dried over anhydrous MgSO4, and evaporated to provide the crude material. The crude material was purified by flash column chromatography using various hexane/ethyl acetate eluent systems to afford the desired products 1a-22a.
References:
Lee, Jongbok;Hong, Myengchan;Jung, Yoonchul;Cho, Eun Jin;Rhee, Hakjune [Tetrahedron,2012,vol. 68,# 8,p. 2045 - 2051] Location in patent:experimental part
588-46-5
142 suppliers
$5.00/100mg

1575-95-7
4 suppliers
inquiry
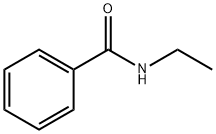
614-17-5
40 suppliers
$172.00/50mg

1575-95-7
4 suppliers
inquiry
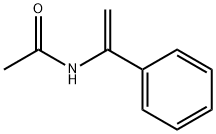
57957-24-1
42 suppliers
$45.00/10mg

1575-95-7
4 suppliers
inquiry