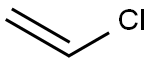
Benzene, 1-(ethenyloxy)-3-methyl- synthesis
- Product Name:Benzene, 1-(ethenyloxy)-3-methyl-
- CAS Number:1005-40-9
- Molecular formula:C9H10O
- Molecular Weight:134.18
Yield:1005-40-9 74%
Reaction Conditions:
with potassium hydroxide semihydrate in dimethyl sulfoxide at 120; under 9562.49 - 11769.2 Torr; for 3 h;Sealed tube;
Steps:
Vinylation of phenols and naphthols with acetylene. General procedure.
General procedure: In the reactor (0.3 L) was charged 50 mmol of ArOH 1, 1.62 g (25 mmol) of KOH*0.5H2O, 50 mL of DMSO, the reactor was closed gas-tight, the air was replaced by nitrogen, and acetylene was charged into the reactor to initial pressure 13-15 at. The reaction mixture at vigorous stirring (240 rpm) was heated at 120°C. The pressure in the reactor at the moment when the temperature reached the desired value was 15-16 at and decreased in the course of the reaction. At the completion of the experiment the reactor was cooled, the reaction mixture was diluted with 1% water solution of NH4Cl (100 mL), the reaction products were extracted with Et2O (6 × 30 mL). The extract was dried with Na2SO4, the solvent was distilled off on a rotary evaporator. The residue was distilled in a vacuum to obtain arylvinyl ethers 2a-2h. Divinyl ethers 4 and 5 were obtained similarly from dihydric phenols (50 mmol) and acetylene.
References:
Trofimov;Oparina;Kolyvanov;Vysotskaya;Gusarova [Russian Journal of Organic Chemistry,2015,vol. 51,# 2,p. 188 - 194][Zh. Org. Khim.,2015,vol. 51,# 2,p. 200 - 206]
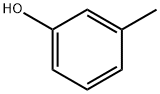
108-39-4
511 suppliers
$13.00/25g

1005-40-9
2 suppliers
inquiry