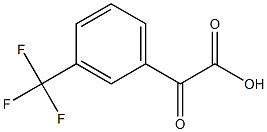
Benzeneacetic acid, a-oxo-3-(trifluoromethyl)- synthesis
- Product Name:Benzeneacetic acid, a-oxo-3-(trifluoromethyl)-
- CAS Number:61560-95-0
- Molecular formula:C9H5F3O3
- Molecular Weight:218.13

124-38-9
122 suppliers
$214.00/14L
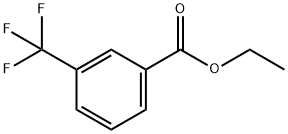
76783-59-0
110 suppliers
$25.00/5G

61560-95-0
18 suppliers
$56.00/250mg
Yield:61560-95-0 58%
Reaction Conditions:
with chloro-trimethyl-silane;magnesium in N,N-dimethyl-formamide at 20;
Steps:
General procedure for reductive carboxylation of ethyl benzoates (1) and benzoylformic acid (2a)
General procedure: A typical procedure is as follows. Magnesium turnings (0.36 g, 15 mmol) for Grignard reagent with no pre-treatment were placed in a 100 mL four-necked flask and were heated to dry. Carbon dioxide was introduced to the flask and a balloon filled with carbon dioxide was attached to the flask. Chlorotrimethylsilane (3.17 mL, 25 mmol) in dry DMF (30 mL) was added to the flask and the reaction mixture was stirred for 30 minutes at room temperature. Ethyl benzoate 1a (0.72 mL, 5 mmol) was added dropwise by means of a syringe and the reaction mixture was stirred at room temperature until the starting material disappeared. Then the reaction mixture was carefully poured into 1 M hydrochloric acid solution (100 mL) and the product was extracted with diethyl ether (40 mL) four times. The combined organic layer was washed with 1 M potassium carbonate solution (40 mL) twice and the product was transferred to the water layer. To the combined water layers, 3 M hydrochloric acid (200 mL) was added and the product was extracted with ether (40 mL) four times. The combined ether layer was washed with water (50 mL) and brine (50 mL), dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to give the product 2a.
References:
Maekawa, Hirofumi;Okawara, Hikaru;Murakami, Taro [Tetrahedron Letters,2017,vol. 58,# 3,p. 206 - 209] Location in patent:supporting information
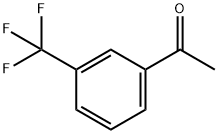
349-76-8
372 suppliers
$10.00/1g

61560-95-0
18 suppliers
$56.00/250mg
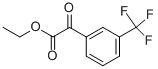
110193-60-7
42 suppliers
$65.00/100mg

61560-95-0
18 suppliers
$56.00/250mg
401-78-5
393 suppliers
$5.00/5g

61560-95-0
18 suppliers
$56.00/250mg