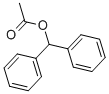
BENZHYDRYL ACETATE synthesis
- Product Name:BENZHYDRYL ACETATE
- CAS Number:954-67-6
- Molecular formula:C15H14O2
- Molecular Weight:226.27
Yield:954-67-6 100%
Reaction Conditions:
with bifunctional polymer in toluene at 20; for 3 h;
Steps:
General Procedure for Esterification Reactions Using 6
General procedure: Acylating reagent 7 (1.2 equiv.) was slowly added dropwise into a stirred solution of alcohol 8 (1.0 equiv.)and polymer 6 in dry toluene (30 mL). The reaction mixture was stirred at room temperature, and whenTLC analysis indicated that 8 had been completely consumed, the reaction mixture was slowly addeddropwise into hexane (150 mL). The resulting precipitate was filtered off and the filtrate was washedwith saturated aqueous Na2CO3 (50 mL). The organic layer was washed with brine (50 mL), dried overanhydrous magnesium sulfate, and concentrated under reduced pressure to afford product ester 9 in anessentially pure state according to 1H NMR analysis. The collected precipitate was dissolved methanol (8mL), and was slowly added dropwise to a vigorously stirred solution of 0.1 N sodium hydroxide inmethanol (100 mL) at 0 C. The resulting light yellow precipitate was filtered, washed with methanol (40mL), and dried under reduced pressure for reuse in the next reaction.
References:
Ma, Shuang;Toy, Patrick H. [Synlett,2016,vol. 27,# 8,art. no. ST-2016-U0018-L,p. 1207 - 1210] Location in patent:supporting information
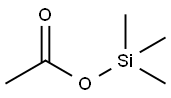
3240-34-4
610 suppliers
$7.00/10g
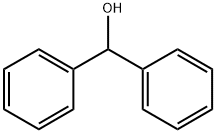
101-81-5
420 suppliers
$7.00/25g

64-19-7
1628 suppliers
$10.00/25ML

954-67-6
5 suppliers
inquiry