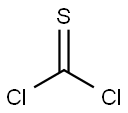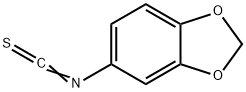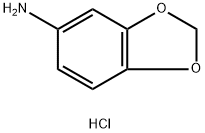
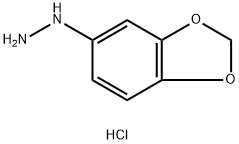
Benzo[d][1,3]dioxol-5-aMine hydrochloride synthesis
- Product Name:Benzo[d][1,3]dioxol-5-aMine hydrochloride
- CAS Number:2620-45-3
- Molecular formula:C7H8ClNO2
- Molecular Weight:173.6
![Benzo[d][1,3]dioxol-5-aMine hydrochloride](/CAS/20150408/GIF/2620-45-3.gif)
2620-45-3
26 suppliers
inquiry
40483-63-4
11 suppliers
inquiry
Yield:40483-63-4 32%
Reaction Conditions:
Stage #1: benzo[1,3]dioxol-5-ylamine; hydrochloridewith hydrogenchloride in water at 20; for 2 h;
Stage #2: with sodium nitrite in water at 0 - 5; for 1.75 h;
Stage #3: with hydrogenchloride;tin(II) chloride dihdyrate in water at 0 - 20; for 12 h;
Steps:
4b
Example 4b (see general route 4, step H, M and K):6-methyl-5H-[l,3]dioxolo[4,5-f| indole[00290] A room temperature solution of benzo[d][l,3]dioxol-5-amine hydrochloride(25.0 g, 144 mmol) in concentrated 12M aqueous hydrochloric acid solution (60 mL) was stirred for two hours, after which, it was cooled to 0 °C to which a solution of sodium nitrite(11.5 g, 167 mmol), in water (50 mL), was added, dropwise over 45 minutes, with internal temperature monitoring such that the reaction temperature does not warm above 5 °C. After stirring for one hour at 0 °C, the reaction mixture was poured slowly into a 0 °C pre-made solution of tin(II) chloride dihydrate (136 g, 605 mmol) in concentrated 12M aqueous hydrochloric acid solution (125 mL). The reaction mixture was allowed to warm up to room temperature, after which it was stored in the freezer overnight (12 hours), leading to the formation of a precipitate. This dark brown solid was successively washed with water (2 x 100 mL) and diethyl ether (3 x 100 mL), and dried to afford benzo[d][l,3]dioxol-5-ylhydrazine hydrochloride (8.60 g, 46.1 mmol, 32% yield) as a dark brown solid. 1H NMR (400 MHz, CD3OD) δ (ppm): 6.79 (d, 1H), 6.63 (d, 1H), 6.50 (dd, 1H), 6.06 (s, 1H), 5.94 (s, 2H).
References:
WO2012/88431,2012,A1 Location in patent:Page/Page column 94-95
![Benzo[d][1,3]dioxol-5-aMine hydrochloride](/CAS/20150408/GIF/2620-45-3.gif)
2620-45-3
26 suppliers
inquiry
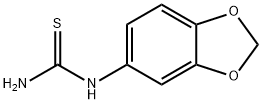
65069-55-8
36 suppliers
$28.00/250mg
![Benzo[d][1,3]dioxol-5-aMine hydrochloride](/CAS/20150408/GIF/2620-45-3.gif)
2620-45-3
26 suppliers
inquiry
![5H-1,3-Dioxolo[4,5-f]indole, 6-methyl-](/CAS/20210305/GIF/74575-44-3.gif)
74575-44-3
0 suppliers
inquiry