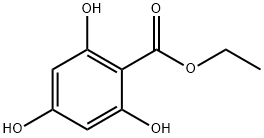
Benzoic acid, 2,4,6-trihydroxy-, ethyl ester synthesis
- Product Name:Benzoic acid, 2,4,6-trihydroxy-, ethyl ester
- CAS Number:90536-74-6
- Molecular formula:C9H10O5
- Molecular Weight:198.17
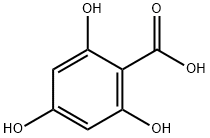
83-30-7
185 suppliers
$24.00/5g
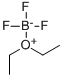
109-63-7
405 suppliers
$10.60/1gm:

90536-74-6
42 suppliers
$136.00/5mg
Yield:90536-74-6 85%
Reaction Conditions:
in ethanol at 20;Heating / reflux;
Steps:
132
2,4,6-trihydroxybenzoic acid (2.00 g, 10 mmol) and CaCl2-distilled ethanol (15.5 mL) were added to a three-necked flask equipped with a reflux condenser, a calcium chloride trap and a rubber septum. Boron-trifluoride-etherate (1.5 mL) was added by syringe to the bright-colored solution, and scant white fumes were observed. The solution was refluxed overnight with stirring at room temperature. The yellow-orange solution was decomposed by addition of water (50 mL) and was extracted with ether (3×50 mL) and ethyl acetate (2×50 mL). Evaporation of the ethyl acetate extract yielded an orange pasty solid containing the desired ethyl trihydroxybenzoate contaminated with phloroglucinol, and other minor side-products (1.2 g). Isolation of the ester was achieved by column chromatography (silica gel 60; 15%-50% ethyl acetate/petroleum ether 60-80). Fractions 12-13 (50% ethyl acetate) were pooled and evaporated to yield 341 mg of 85% pure (reverse-phase HPLC, 70% acetonitrile in H2O, 1 mL/min, 256 nm, Rt=2.25') ethyl trihydroxybenzoate (NMR). This material was dried in-vacuo and subjected to a hoesch condensation (procedure by Whalley, J.Chem Soc., 1951, 3229). 340 mg (1.5 mmol) were placed in a flame-dried air-jacketed 2-necked flask equipped with an in-situ HCl gas-generating system (H2SO4 equal pressure funnel. Kipp apparatus with NH4Cl, connected in tandem to H2SO4 and air traps) was dissolved in 50 mL sodium dried ether. Oven-dried ZnCl2 (0-8 g) and AlCl3, (anhydrous, under argon) were added to the clear solution. Upon addition of AlCl3 a vigorous reaction ensued and the solution turned immediately turbid yellow. Acetonitrile (HPLC grade; 2 mL, 38 mmol) was added and a dry stream of dry hydrogen chloride gas was passed through the mixture. The solution became clear within a few minutes, was turbid again after one hour and was left overnight at room temperature. The ethereal solution was filtered in-vacuo and the white solid (ketimine hydrochloride) was dissolved in water (25 mL) and hydrolysed by heating on a hot plate. The aqueous solution was concentrated to 5 mL and cooled. Upon cooling, needles appeared which were kept overnight at 4° C. Cold filtration yielded sweet-odored yellow needles, which were dried intensively in a flash evaporator (12 mg). The material was 72% pure by reverse-phase HPLC (70% acetonitrile in H2O, 1 mL/min, 256 nm, Rt=3.50'). 1H NMR (CD3OD, 200 MHz): δ5.97 (s, 1H), 4.13 (q, 2H, J=7.0 Hz), 2.63 (s, 3 H), 1.51 (t, 3 H, J=7.0 Hz).
References:
US6828350,2004,B1 Location in patent:Page/Page column 17