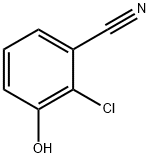
Benzonitrile, 2-chloro-3-hydroxy- synthesis
- Product Name:Benzonitrile, 2-chloro-3-hydroxy-
- CAS Number:51786-11-9
- Molecular formula:C7H4ClNO
- Molecular Weight:153.57
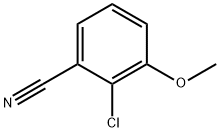
853331-52-9

51786-11-9
General procedure for the synthesis of 2-chloro-3-hydroxybenzonitrile from 2-chloro-3-methoxybenzonitrile: Iodocyclohexane (3.86 mL, 29.83 mmol) was slowly added to a solution of DMF (10 mL) containing 2-chloro-3-methoxybenzonitrile (1 g, 5.97 mmol) at 20 °C under nitrogen protection. The reaction mixture was heated to 155 °C with continuous stirring for 7 hours. After the reaction was completed, it was cooled to room temperature and the mixture was poured into water. Extraction was carried out with ethyl acetate (2 x 50 mL), the organic phases were combined and washed with brine (50 mL), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give the crude product. The crude product was ground with dichloromethane and the resulting solid was collected by filtration and dried under vacuum to give 2-chloro-3-hydroxybenzonitrile (0.510 g, 55.7% yield) as a white solid. No additional product was obtained from the filtrate treatment. The product was confirmed by 1H NMR (400 MHz, DMSO): δ 7.25-7.39 (3H, m), 11.00 (1H, s). The mass spectrum (ES-) showed m/z (M-H)- = 152.
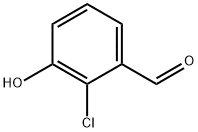
56962-10-8
110 suppliers
$21.00/250mg

51786-11-9
59 suppliers
inquiry
Yield:51786-11-9 100%
Reaction Conditions:
with hydroxylamine hydrochloride;acetic acid for 2 h;Heating / reflux;
Steps:
72
Preparation 72: 2-Chloro-3-hydroxybenzonitrile A mixture of 2-chloro-3-hydroxybenzaldehyde [(2 g, 12.8 mmol) WO 2005007633, p34] and hydroxylammonium chloride (1.33 g, 19.6 mmol) in acetic acid (20 mL) was heated under reflux for 2 hours. The reaction mixture was then cooled to room temperature and partitioned between diethyl ether and water. The organic layer was separated, washed with brine, dried over magnesium sulfate and concentrated in vacuo to give a white solid. The solid was then dissolved in ethyl acetate washed with water and brine, dried over magnesium sulfate and concentrated in vacuo to afford the title compound as a solid in quantitative yield. 1H NMR(400 MHz, CD3OD) δ: 7.18(d, 1H), 7.26(m, 2H)
References:
US2006/160786,2006,A1 Location in patent:Page/Page column 36

853331-52-9
28 suppliers
$54.00/250mg

51786-11-9
59 suppliers
inquiry
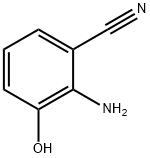
211172-52-0
40 suppliers
inquiry

51786-11-9
59 suppliers
inquiry
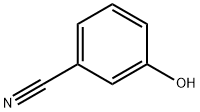
873-62-1
261 suppliers
$6.00/1g

51786-11-9
59 suppliers
inquiry
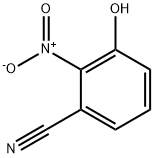
129298-23-3
34 suppliers
inquiry

51786-11-9
59 suppliers
inquiry