
Benzonitrile, 4,4'-(2,5-thiophenediyl)bis- synthesis
- Product Name:Benzonitrile, 4,4'-(2,5-thiophenediyl)bis-
- CAS Number:55368-38-2
- Molecular formula:C18H10N2S
- Molecular Weight:286.35
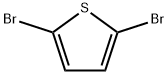
3141-27-3
309 suppliers
$5.00/10g
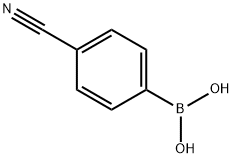
126747-14-6
408 suppliers
$6.00/1g

55368-38-2
19 suppliers
inquiry
Yield:55368-38-2 94%
Reaction Conditions:
with palladium diacetate;sodium carbonate;triphenylphosphine in 1,2-dimethoxyethane;water at 80; for 2 h;Inert atmosphere;Microwave irradiation;
Steps:
5.1.2. 4,4'-Thiene-2,5-diyldibenzonitrile (2)
A reaction vessel containing a solution of Pd(OAc)2 (20 mg, 0.09 mmol) and PPh3 (94.5 mg, 0.36 mmol) in DME (9 mL) was stirred for10 min under Ar atmosphere. Following, 2,5-dibromothiophene (0.1 mL, 0.89 mmol) and 2 M Na2CO3 (1.6 mL, 3.2 mmol) were added. After 5 min, 4-cyanophenylboronic acid (290 mg, 1.97 mmol) was added, and the mixture was stirred for 2 h at 80 °C in a MW reactor. The solvent was then removed under reduced pressure, and the reaction mixture was suspended in dichloromethane (DCM), transferred to a separation funnel, and washed well with saturated Na2CO3 solution (2 × 25 mL) containing 1 mL NH3. The organic layer was collected, dried with anh. Na2SO4, and the solvent was filtered. Subsequently, the solvent was removed under reduced pressure and intermediate 2 was isolated using column chromatography (dry-flash, SiO2, eluent DCM), and collected as a pale yellow amorphous powder. The yield was 240 mg (94%, mp = 282-284 °C) [21]. IR (KBr): 2925w, 2359w, 1535m, 1506m, 1380w, 1207s, 1150s, 1035s, 825s, 781m, 732m, 702m cm-1. 1H NMR (200 MHz, CD3OD): 7.80-7.60 (m, 8H-Ar), 7.50-7.45 (s, H-C(3) and H-C(4)). 13C NMR (50 MHz, CD3OD): 143.31, 137.90, 132.88, 126.36, 125.98, 118.64, 111.19. HPLC purity: method A, RT 15.397 min, area 99.03%; method B: RT 14.656 min, area 96.58%.
References:
Opsenica, Igor;Filipovic, Vuk;Nuss, Jon E.;Gomba, Laura M.;Opsenica, Dejan;Burnett, James C.;Gussio, Rick;Solaja, Bogdan A.;Bavari, Sina [European Journal of Medicinal Chemistry,2012,vol. 53,p. 374 - 379] Location in patent:experimental part
18245-28-8
108 suppliers
$10.00/1g
623-00-7
489 suppliers
$6.00/10g

15961-46-3
3 suppliers
inquiry

55368-38-2
19 suppliers
inquiry
![Benzonitrile, 4-[5-(trimethylsilyl)-2-thienyl]-](/CAS/20210305/GIF/1226978-77-3.gif)
1226978-77-3
1 suppliers
inquiry
188290-36-0
2 suppliers
inquiry

623-00-7
489 suppliers
$6.00/10g

15961-46-3
3 suppliers
inquiry

55368-38-2
19 suppliers
inquiry

3141-27-3
309 suppliers
$5.00/10g
171364-82-2
173 suppliers
$6.00/250mg

55368-38-2
19 suppliers
inquiry

18245-28-8
108 suppliers
$10.00/1g

623-00-7
489 suppliers
$6.00/10g

15961-46-3
3 suppliers
inquiry

55368-38-2
19 suppliers
inquiry
1591-30-6
157 suppliers
$44.00/1g
![Benzonitrile, 4-[5-(trimethylsilyl)-2-thienyl]-](/CAS/20210305/GIF/1226978-77-3.gif)
1226978-77-3
1 suppliers
inquiry