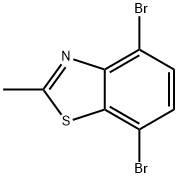
Benzothiazole, 4,7-dibromo-2-methyl- synthesis
- Product Name:Benzothiazole, 4,7-dibromo-2-methyl-
- CAS Number:1417896-97-9
- Molecular formula:C8H5Br2NS
- Molecular Weight:307.01

110704-51-3
5 suppliers
inquiry

1417896-97-9
3 suppliers
inquiry
Yield:1417896-97-9 48%
Reaction Conditions:
with sodium hydroxide;potassium hexacyanoferrate(III) in ethanol;water at 95; for 2 h;regiospecific reaction;
Steps:
4 Synthesis of 4,7-dibromo-2-methylbenzo[d]thiazole (5)
A mixture of compound 4 (3.09 g, 10 mmol) and sodium hydroxide (3.2 g, 80 mmol) in water-ethanol (40 mL-2 mL) was added dropwise to a solution of potassium ferricyanide (13.17 g, 40 mmol) in water (20 mL) and stirred at 95 °C for 2 h. The reaction mixture was cooled in an ice bath to yield the precipitate. Subsequently, the precipitate was filtered, washed with water, and concentrated in vacuo. The crude product was purified by column chromatography (petroleum ether/ethyl acetate = 20/1, v/v) to obtain the desired product 5 as pale yellow powder (1.46 g, 48% yield). mp 153-155 °C. IR (KBr): 3080, 2919, 1856, 1522, 1446, 1372, 1339, 1170, 1113, 1087, 909, 798. 1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 8.4 Hz, 1H), 7.34 (d, J = 8.4 Hz, 1H), 2.88 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 168.7, 151.1, 139.2, 130.4, 128.4, 115.0, 112.9, 20.7. HRMS (EI, m/z): calcd. for C8H5NSBr2, 304.8509 [M]+; found, 304.8509.
References:
Ci, Zhenhua;Yu, Xiaoqiang;Bao, Ming;Wang, Chaolei;Ma, Tingli [Dyes and Pigments,2013,vol. 96,# 3,p. 619 - 625]
106-37-6
479 suppliers
$9.00/5g

1417896-97-9
3 suppliers
inquiry
3460-18-2
299 suppliers
$6.00/10g

1417896-97-9
3 suppliers
inquiry
3638-73-1
208 suppliers
$10.00/1g

1417896-97-9
3 suppliers
inquiry
25462-66-2
21 suppliers
$155.53/250mg

1417896-97-9
3 suppliers
inquiry