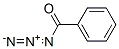
benzoylazide synthesis
- Product Name:benzoylazide
- CAS Number:582-61-6
- Molecular formula:C6H5CON3
- Molecular Weight:0
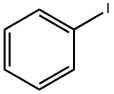
93-97-0
385 suppliers
$6.00/25g
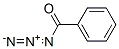
582-61-6
6 suppliers
inquiry
Yield: 84%
Reaction Conditions:
with sodium azide in acetonitrile for 0.666667 h;
Steps:
3.5. General Procedure for the Preparation of Acyl AzideFrom Produced Carboxylic Anhydrides and Sodium Azide
General procedure: After the completion of the synthesis of carboxylic anhydride(the TsCl disappeared completely, monitored by TLC),NaN3 (0.7 mmol, 0.05 g) and CH3CN (2 mL) were added tothe reaction mixture and the mixture was stirred at room temperature for the appropriate time (Table 6) until the carboxylic anhydride disappeared completely (monitored byTLC). After the completion of the reaction, CH2Cl2 or Et2O(2×10 mL) was added to the reaction mixture, stirred, filtered,and the organic layer was dried over CaCl2. The evaporation of the solvent afforded a highly pure product.
References:
Eskandari, Parvin;Kazemi, Foad [Letters in Organic Chemistry,2017,vol. 14,# 6,p. 431 - 439]
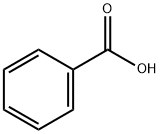
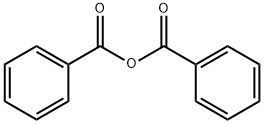
65-85-0
1437 suppliers
$10.00/25 g
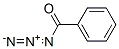
582-61-6
6 suppliers
inquiry
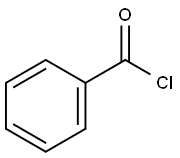
98-88-4
598 suppliers
$10.00/5g
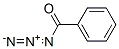
582-61-6
6 suppliers
inquiry
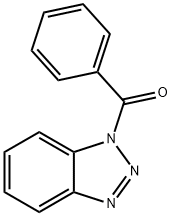
4231-62-3
38 suppliers
$83.40/1g
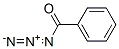
582-61-6
6 suppliers
inquiry