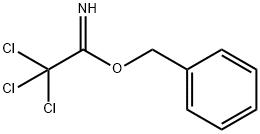
BENZYL 2,2,2-TRICHLOROACETIMIDATE synthesis
- Product Name:BENZYL 2,2,2-TRICHLOROACETIMIDATE
- CAS Number:81927-55-1
- Molecular formula:C9H8Cl3NO
- Molecular Weight:252.52
545-06-2

100-51-6

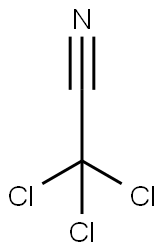
81927-55-1
DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) (1.24 mL, 8.31 mmol) was slowly added dropwise to a solution of dichloromethane (86 mL) containing benzyl alcohol (8.60 mL, 83.11 mmol) and trichloroacetonitrile (41.66 mL, 415.57 mmol), and the reaction mixture was cooled under ice bath conditions to 0 °C . Subsequently, the mixture was gradually warmed up to room temperature reaction. The progress of the reaction was monitored by thin layer chromatography (TLC) (petroleum ether/ethyl acetate, 95:5, V/V) until the formation of trichloroacetimide was observed. After 3 h of reaction, the solvent was removed by distillation under reduced pressure and the resulting residue was purified by silica gel column chromatography (eluent: petroleum ether/ethyl acetate, 95:5, V/V), followed by distillation at 80 °C/0.3 mbar to afford the target product 2,2,2-trichloroacetimidobenzyl ester as a colorless oil in 94% yield.TLC Rf value: 0.27 (petroleum ether/ethyl acetate. 95:5, V/V).1H NMR (400.13 MHz, CDCl3) δ (ppm): 5.26 (s, 2H, H1'); 7.24-7.36 (m, 5H, HPh); 8.31 (wide s, 1H, =NH).13C NMR (100.62 MHz, CDCl3) δ (ppm): 70.7 (Cl'); 91.3 (C2); 127.7, 128.3 and 128.5 (5C, CPh); 134.4 (C2'); 162.5 (C1).

545-06-2
243 suppliers
$10.00/5g

100-51-6
1433 suppliers
$5.00/100g

81927-55-1
161 suppliers
$11.19/5G
Yield:81927-55-1 100%
Reaction Conditions:
with 1,8-diazabicyclo[5.4.0]undec-7-ene in n-heptane at 0; for 0.25 h;Solvent;
Steps:
Synthesis of Bn-TCAI (1) at a 1 g scale
To a suspension of BnOH (1.00 g, 9.28 mmol) and Cl3CCN (1.47 g, 10.2 mmol) in heptane (23 mL) was added DBU(141 mg, 926 μmol) at 0 °C. After the suspension changed to be a solution (actual reaction time = 15 min), heptane (23mL) and saturated aq NH4Cl (23 mL) were added to the reaction mixture. The separated heptane layer was washed withsaturated aq NH4Cl (23 mL). The general drying procedure gave Bn-TCAI (1) (2.34 g, 100%) as colorless oil. The NMRspectra of 1 were in good agreement with the literature data.1
References:
Ikeuchi, Kazutada;Murasawa, Kentaro;Yamada, Hidetoshi [Synlett,2019,vol. 30,# 11,p. 1308 - 1312] Location in patent:supporting information