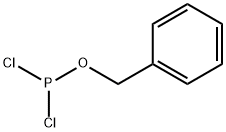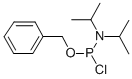
BENZYL-N,N-DIISOPROPYLCHLOROPHOSPHORAMIDITE synthesis
- Product Name:BENZYL-N,N-DIISOPROPYLCHLOROPHOSPHORAMIDITE
- CAS Number:128753-68-4
- Molecular formula:C13H21ClNOP
- Molecular Weight:273.74
Yield:128753-68-4 82%
Reaction Conditions:
with triethylamine in diethyl ether at -20 - 20;Inert atmosphere;
Steps:
2.2 Benzyl-N,N-diisopropylchlorophosphoramidite (3)
To a cooled solution of PCl3 (59.2 mL, 682 mmol) in CH2Cl2 (200 mL) at 0 °C was added a solution of benzyl alcohol (14.2 mL, 136 mmol) in CH2Cl2 (50 mL) dropwise over 3 h under an atmosphere of argon.
The reaction was stirred at 20 °C for 2 h.
Concentration in vacuo afforded 8 as light yellow oil (28.5 g, 99%).
To a solution of 8 (28.5 g, 134 mmol) in ether (120 mL) was added a solution of diisopropylamine (19.3 mL, 136 mmol) and triethylamine (19.0 mL, 136 mmol) in ether (50 mL) dropwise under an atmosphere of argon at -20 °C over 3 h.
The reaction was stirred overnight at 20 °C.
The triethylammonium chloride was removed by filtration, and the filtrate was concentrated in vacuo.
Vacuum distillation (90 °C, 8 mm Hg) of the residue oil afforded 3 (28.4 g, 82%) as colorless oil; 1H NMR (400 MHz, CDCl3): δ 7.37 (s, 4H), 7.33-7.32 (m, 5H), 4.99-4.91 (m, 2H), 3.90-3.84 (m, 2H), 1.35 (d, J = 6.0 Hz, 6H), 1.27 (d, J = 5.6 Hz, 6H) ppm; 13C NMR (100 MHz, CDCl3): δ 137.5, 128.5, 127.9, 127.3, 67.6, 46.1, 46.0, 24.1, 23.3 ppm; 31P NMR (161 MHz, CDCl3): δ 181.57 ppm.
References:
Sun, Qi;Yang, Qingkun;Gong, Shanshan;Fu, Quanlei;Xiao, Qiang [Bioorganic and Medicinal Chemistry,2013,vol. 21,# 21,p. 6778 - 6787]

100-51-6
1355 suppliers
$5.00/100g

128753-68-4
12 suppliers
inquiry
108-18-9
351 suppliers
$10.00/1g

100-51-6
1355 suppliers
$5.00/100g

128753-68-4
12 suppliers
inquiry