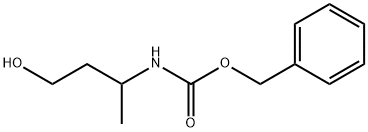
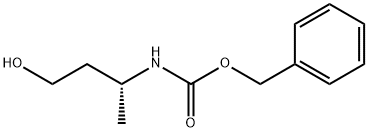
benzyl (S)-(4-hydroxybutan-2-yl)carbamate synthesis
- Product Name:benzyl (S)-(4-hydroxybutan-2-yl)carbamate
- CAS Number:168827-91-6
- Molecular formula:C12H17NO3
- Molecular Weight:223.27
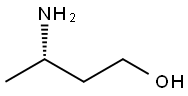
878999-34-9
1 suppliers
inquiry

168827-91-6
9 suppliers
inquiry
Yield:168827-91-6 87%
Reaction Conditions:
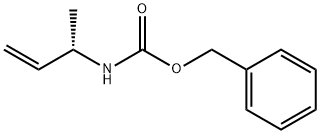
Stage #1: (1-(S)-methyl-allyl)carbamic acid benzyl esterwith 9-bora-bicyclo[3.3.1]nonane in tetrahydrofuran at 20; for 16 h;
Stage #2: with dihydrogen peroxide;sodium hydroxide in tetrahydrofuran;water at 0;
Steps:
58
(1-(S)-Methyl-allyl)-carbamic acid benzyl ester (1.96 g, 9.56 mmol) was taken up in THF (200 mL). 9-BBN 0.5M in THF (38 mL, 19.0 mmol) was added slowly to the reaction mixture. The solution was allowed to stir at rt for 16 h. Quenched with H2O (65 mL), followed by 1.0M NaOH(aq) (20 mL). The solution was cooled to 0° C. in an ice bath and H2O2 30% in H2O (7.0 mL) was slowly added. The reaction was partitioned between Et2O and H2O. The layers were separated and the aqueous washed with Et2O. The combine organics were dried over Na2SO4. Solids were filtered and the solvent removed under reduced pressure. (3-hydroxy-1-(S)-methyl-propyl)-carbamic acid benzyl ester (1.85 g, 87%) was isolated by silica gel chromatography as a white solid.
References:
US2011/20278,2011,A1 Location in patent:Page/Page column 56-57
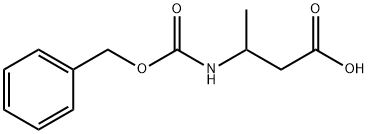
51440-81-4
22 suppliers
$85.00/250mg
866395-21-3
8 suppliers
inquiry

168827-91-6
9 suppliers
inquiry