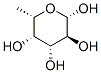
beta-L-fucose synthesis
- Product Name:beta-L-fucose
- CAS Number:13224-93-6
- Molecular formula:C6H12O5
- Molecular Weight:164.16
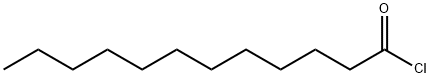
112-16-3
321 suppliers
$13.00/50g
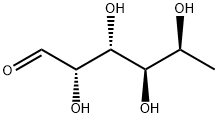
2438-80-4
479 suppliers
$9.00/250mg

13224-93-6
4 suppliers
inquiry
Yield:-
Reaction Conditions:
with pyridine;dmap in pentane;
Steps:
B Synthesis of 1,2,3,4,6-Penta-O-lauroyl-α/β-L-fucopyranoside 1
Example B Synthesis of 1,2,3,4,6-Penta-O-lauroyl-α/β-L-fucopyranoside 1 To a suspension of L-fucose (11.8 g, 71.8 mmol), pyridine (28.4 g, 358 mmol), and 4-dimethylaminopyridine (cat.) in pentane (400 mL) under argon atmosphere, was added lauroyl chloride (78.5 g, 358 mmol) at -78° C. The mixture was allowed to reach ambient temperature. The resulting white slurry slowly dissolved and a fine precipitate of pyridinium hydrochloride formed. After 24 h, the pyridinium hydrochloride was filtered off and the pentane solution was concentrated. Column chromatography (SiO2, pentane/EtOAc 15:1) gave 1 (68 g, quant. yield). 1 H-NMR for the α-anomer: δ 6.35 (d, 1H, J 2.9 Hz, H-1), 5.34 (m, 3H, H-2, H-3, H4), 4.26 (br q, 1H, J 6.2 Hz, H-5), 2.42 (t, 3H, J 7.5 Hz, --CH2 CO--), 2.37 (t, 3H, J 7.5 Hz, --CH2 CO--), 2.21 (m, 6H, --CH2 CO--), 1.14 (d, 3H, J 6.5 Hz, H-6), 0.88 (t, 12H, J 7.0 Hz, --CH2 CH3). 1 H-NMR for the β-anomer: δ 5.69 (d, 1H, J 8.2 Hz, H-1), 5.32 (dd, 1H, J 8.2 and 10.4 Hz, H-2), 5.28 (dd, 1H, J 1.0 and 3.2 Hz, H-4), 5.08 (dd, 1H, J 3.4 and 10.4 Hz, H-3), 3.95 (br q, 1H, J 6.6 Hz, H-5), 2.42 (br t, 3H, J 7.5 Hz, --CH2 CO--), 2.34 (dt, 3H, J 4.5 and 7.5 Hz, --CH2 CO--), 2.21 (m, 6H, --CH2 CO--), 1.21 (d, 3H, J 6.5 Hz, H-6), 0.88 (t, 12H, J 7.0 Hz, --CH2 CH3).
References:
US6087339,2000,A

67-56-1
782 suppliers
$9.00/25ml
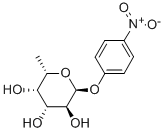
10231-84-2
143 suppliers
$33.00/10mg
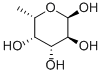
6696-41-9
48 suppliers
$23.30/1G

13224-93-6
4 suppliers
inquiry
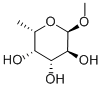
14687-15-1
88 suppliers
$44.79/250MG
