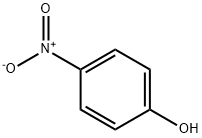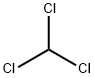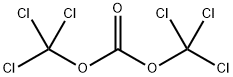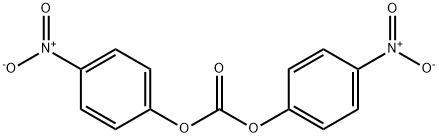
BIS(4-NITROPHENYL) CARBONATE synthesis
- Product Name:BIS(4-NITROPHENYL) CARBONATE
- CAS Number:5070-13-3
- Molecular formula:C13H8N2O7
- Molecular Weight:304.21
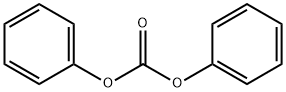
102-09-0

5070-13-3
Example 1: Nitration of diphenyl carbonate in the presence of nitrobenzene. To a 2000 mL reactor equipped with a mechanical stirrer, thermometer and jacketed charging funnel was added 107.11 g of diphenyl carbonate (0.5 mol) and 500 mL of nitrobenzene. The mixture was stirred until completely dissolved. Meanwhile, a mixed acid reagent was prepared by mixing 99.02 g of concentrated nitric acid and 125 mL of concentrated sulfuric acid in a beaker under cooling conditions. The cooled mixed acid reagent was transferred to a jacketed charging funnel and further cooled in an ice bath. After diphenyl carbonate was completely dissolved, the temperature of the reaction mixture was adjusted to 20 °C using an ice/water bath. The mixed acid reagent was slowly added dropwise at a rate that maintained the reaction temperature near 20°C for a total dropwise time of about 60 minutes. After the dropwise addition was completed, stirring of the reaction mixture was continued for 60 minutes. Subsequently, the reaction mixture was quenched by pouring the reaction mixture into 600 mL of ice/water mixture. 600 mL of ethyl acetate was added and the mixture was transferred to a dispensing funnel and shaken well. The organic layer was separated and the aqueous layer was extracted three times with 100 mL of ethyl acetate. The organic layers were combined and washed sequentially with 200 mL saturated sodium bicarbonate solution and 200 mL saturated brine. The organic layers were dried over anhydrous sodium sulfate and the ethyl acetate was removed by rotary evaporator. The nitrobenzene solution of the crude product was slowly poured into 2000 mL of cyclohexane and the precipitated crude product (215.16 g) was collected by filtration. Gas chromatographic analysis showed the following product composition: 95.6% 4,4'-dinitrodiphenyl carbonate, 0.6% 4,3'-dinitrodiphenyl carbonate, and 3.8% 4,2'-dinitrodiphenyl carbonate. The crude product was recrystallized by mixed solvent recrystallization from toluene and cyclohexane to give 142.7 g of bis(4-nitrophenyl) carbonate in 94% yield.

102-09-0
415 suppliers
$6.00/25g

5070-13-3
253 suppliers
$5.00/5g
Yield:5070-13-3 94%
Reaction Conditions:
with mixed-acid;sulfuric acid;nitric acid in cyclohexane;ethyl acetate;nitrobenzene
Steps:
1 Example 1
Example 1 Nitration of diphenyl carbonate in the presence of nitrobenzene. A 2000 ml reactor equipped with a mechanical stirrer, thermometer, and a jacketed addition funnel was charged with 107.11 g of diphenyl carbonate (0.5 mol) and 500 ml of nitrobenzene. The mixture was stirred until dissolution was complete. In the meantime, the mixed acid reagent was prepared by mixing 99.02 g of concentrated nitric acid and 125 ml of concentrated sulfuric acid in a beaker with cooling. The cooled mixed-acid reagent was then poured into the jacketed addition funnel and further cooled with ice. After the diphenyl carbonate had dissolved in the reaction vessel, the temperature was adjusted to 20° C. with an ice/water bath. The mixed-acid reagent was added at a rate which maintained the temperature of the reaction mixture at close to 20° C. The total addition took around 60 min. Following the addition, the reaction was stirred for an additional 60 min. The reaction mixture was then poured over 600 ml of ice/water. A 600 ml portion of ethyl acetate was added and the mixture transferred to a separatory funnel and shaken. The organic layer was removed and the aqueous layer was washed 3 times with 100 ml portions of ethyl acetate. The organic layers were combined and washed with 200 ml of saturated sodium bicarbonate and 200 ml of saturated brine. The organic layer was then dried over sodium sulfate. After the reaction mixture was dried, the ethyl acetate was removed by rotatory evaporation. The nitrobenzene solution of crude product was poured into 2000 ml of cyclohexane and the precipitated crude product (215.16 g) recovered by filtration. Gas chromatography showed the isomeric carbonates to be present in 95.6%, 4,4'-, 0.6%, 4,3'-, and 3.8%, 4,2'-dinitrodiphenyl carbonate, amounts. Recrystallization from a mixture of toluene and cyclohexane resulted in 142.7 g of di(4-nitrophenyl)carbonate or a 94% yield.
References:
Amoco Corporation US5037994, 1991, A
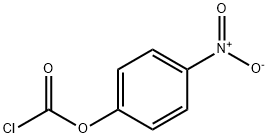
7693-46-1
434 suppliers
$18.00/5g

5070-13-3
253 suppliers
$5.00/5g

7693-46-1
434 suppliers
$18.00/5g
602-09-5
350 suppliers
$6.00/5g

5070-13-3
253 suppliers
$5.00/5g
![Dinaphtho[2,1-d:1',2'-f][1,3]dioxepin-8-one](/CAS/20240320/GIF/138537-48-1.gif)
138537-48-1
0 suppliers
inquiry