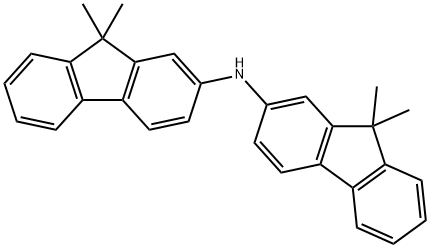
Bis-(9,9-diMethyl-9H-fluoren-2-yl)-aMine synthesis
- Product Name:Bis-(9,9-diMethyl-9H-fluoren-2-yl)-aMine
- CAS Number:500717-23-7
- Molecular formula:C30H27N
- Molecular Weight:401.54
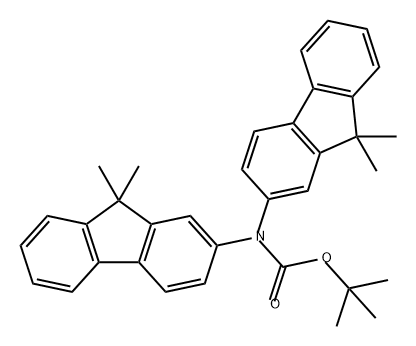
1185882-60-3

500717-23-7
The general procedure for the synthesis of bis(9,9-dimethyl-9H-fluoren-2-yl)amine from the compound (CAS: 1185882-60-3) was as follows: 0.4 g of tert-butoxycarbonyl-protected arylamine 1 (0.8 mmol) was dissolved in 1 mL of tetrahydrofuran and 8 mL of trifluoroacetic acid (TFA). The reaction mixture was stirred at room temperature for 10 min and the color of the solution was observed to change to dark green. Subsequently, TFA was removed by evaporation under vacuum. dichloromethane was added to the residue and neutralized with saturated aqueous sodium hydroxide solution. The mixture was extracted using dichloromethane and the organic layers were combined and dried over anhydrous sodium sulfate. Purification by column chromatography (stationary phase: silica gel, mobile phase: ethyl acetate/hexane = 1:10, v/v) afforded 0.3 g of light yellow target compound 2 in 95% yield. Melting point: 178°C. 1H NMR (300 MHz, (CD3)2CO): δ 7.7-7.67 (m, 4H), 7.48 (d, J = 6.9 Hz, 2H), 7.36 (s, 2H), 7.31-7.22 (m, 4H), 7.18 (d, J = 7.2 Hz, 2H), 1.46 (s, 12H). 13C NMR (300 MHz, (CD3)2CO): δ 155.97, 153.83, 144.45, 140.29, 132.48, 127.79, 126.73, 123.27, 121.67, 119.69, 117.32, 112.46, 47.28, 27.53.

1185882-60-3
0 suppliers
inquiry

500717-23-7
156 suppliers
$5.00/100mg
Yield:500717-23-7 95%
Reaction Conditions:
Stage #1: tert-butyl bis(9,9-dimethyl-9H-fluoren-7-yl)carbamatewith trifluoroacetic acid in tetrahydrofuran at 20; for 0.166667 h;
Stage #2: with sodium hydroxide in tetrahydrofuran;dichloromethane;water;
Steps:
1
Bis(9,9-dimethyl-9H-fluoren-7-yl)amine (B) 0.4 g of tert-Butoxycarbonyl-protected arylamine 1 (0.8 mmol) was dissolved in 1 ml of tetrahydrofurane, and 8 ml of trifluoroacetic acid (TFA) was added to the resulting mixture. Then, the reaction mixture was stirred at room temperature for 10 minutes, thereby obtaining a solution having a color that turned dark green. TFA was evaporated in a vacuum, dichloromethane was added to the resultant, and the resultant was neutralized using an aqueous saturated sodium hydroxide solution. The mixture was extracted with solid dichloromethane solutions as described above, and the remaining moisture in the organic layer was removed. Then, column chromatography (stationary phase: silica gel, mobile phase: ethyl acetate:hexane=1:10 volume ratio) was performed on the resultant solution to obtain 0.3 g of pale yellow Compound 2 at a yield of 95%. Mp: 178 °C 1H NMR (300 MHz, (CD3)2CO):δ 7.7-7.67 (m, 4H), 7.48 (d, J=6.9 Hz), 7.36 (s, 2H), 7.31-7.22 (m, 4H), 7.18 (d, 2H J=7.2 Hz), 1.46 (s, 12H). 13C NMR(300MHz,(CD3)2CO):δ 155.97, 153.83, 144.45, 140.29, 132.48, 127.79, 126.73, 123.27, 121.67, 119.69, 117.32, 112.46, 47.28, 27.53.
References:
EP2371813,2011,A1 Location in patent:Page/Page column 13
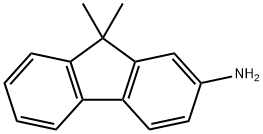
108714-73-4
305 suppliers
$6.00/1g
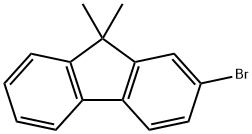
28320-31-2
311 suppliers
$5.00/1g

500717-23-7
156 suppliers
$5.00/100mg
144981-85-1
180 suppliers
$7.00/1g

500717-23-7
156 suppliers
$5.00/100mg