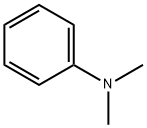
N,N-Dimethylaniline synthesis
- Product Name:N,N-Dimethylaniline
- CAS Number:121-69-7
- Molecular formula:C8H11N
- Molecular Weight:121.18

124-38-9
134 suppliers
$214.00/14L
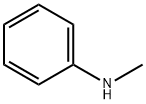
100-61-8
400 suppliers
$8.00/1g

121-69-7
567 suppliers
$10.00/25mL
Yield:121-69-7 99%
Reaction Conditions:
with [Ru(Triphos)(TMM)];hydrogen;1,1,1-trifluoro-N-(trifluoromethylsulfonyl)methanesulfonamide in tetrahydrofuran at 150; under 60006 Torr; for 10 h;Inert atmosphere;Temperature;Time;Reagent/catalyst;Pressure;
Steps:
I
General procedure: General procedures for direct methylation of amines with CO2/H2. All high pressure batch experiments were conducted in stainless steel 10 mL autoclaves equipped with a glass inlet and a magnetic stir bar. Prior to use, the autoclave was dried under vacuum for 3 hours and repeatedly filled with argon. Under an argon atmosphere, catalyst [Ru(Triphos)(TMM)] (0.019 g, 0.025 mmol) and HNTf2 (0.014 g, 0.05 mmol) were weighed in a Schlenk tube. After dissolving in THF (1.0 mL), the mixture was transferred via cannula to the autoclave followed by the addition of the aniline substrate (1.0 mmol) in THF (1.0 mL). The autoclave was then pressurized with CO2 to 20 bar and then H2 was added up to a total pressure of 80 bar. The reaction mixture was stirred and heated to 150 °C in an oil bath. After lOh, the autoclave was cooled in an ice bath and then carefully vented. The reaction solution was analyzed by 'H-NMR with internal standard mesitylene and the results confirmed by gas chromatography using dodecane as internal standard. EXAMPLE I: Methylation of N-methylaniline to N,N-Dimethylaniline The reaction was carried out as described above, except where indicated. The results are shown in the following Table I: TABLE I Entry Acid Temp [C°] Time [h] Yield [%] [mol%] 1 - 140 10 2 2 HNTf2 (0.5) 140 22 11 3 HNTf, (2.5) 140 22 84 4 HNTf, (5), no catalyst added 140 22 0 5 HNTf2 (5), 1% catalyst used 140 10 50 6 HNTf2 (5) 140 22 97 7 HNTf2 (5), 140 22 25 C02/H2: (10/30 bar) 8 HNTf, (5) 120 22 81 9 HNTf, (5) 100 22 58 10 HNTf, (5) 150 0.5 9 11 HNTf, (5) 150 1 20 12 HNTf, (5) 150 1.5 29 13 HNTf, (5) 150 2 34 14 HNTf, (5) 150 3 49 15 HNTf, (5) 150 4 69 16 HNTf, (5) 150 8 97 17 HNTf, (5) 150 10 99 18 MSA (5) 150 10 95 19 / TsOH (5) 150 10 97
References:
WO2015/753,2015,A1 Location in patent:Page/Page column 7-8
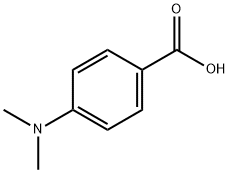
619-84-1
354 suppliers
$5.00/10g

121-69-7
567 suppliers
$10.00/25mL
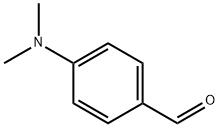
100-10-7
633 suppliers
$5.00/1g

121-69-7
567 suppliers
$10.00/25mL

124-38-9
134 suppliers
$214.00/14L
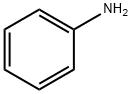
62-53-3
688 suppliers
$10.00/1g

121-69-7
567 suppliers
$10.00/25mL
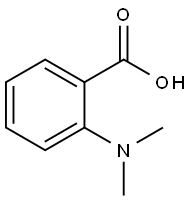
610-16-2
102 suppliers
$7.00/100mg

121-69-7
567 suppliers
$10.00/25mL