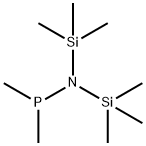
BIS(TRIMETHYLSILYL)AMIDODIMETHYLPHOSPHINE synthesis
- Product Name:BIS(TRIMETHYLSILYL)AMIDODIMETHYLPHOSPHINE
- CAS Number:63744-11-6
- Molecular formula:C8H24NPSi2
- Molecular Weight:221.43
Yield:-
Reaction Conditions:
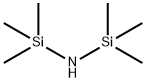
Stage #1: 1,1,1,3,3,3-hexamethyl-disilazanewith n-butyllithium in diethyl ether;hexane at 20; for 2 h;Cooling with ice;Inert atmosphere;
Stage #2: with phosphorus trichloride in diethyl ether;hexane;isopropyl alcohol at -70 - 0; for 1 h;Inert atmosphere;
Stage #3: methylmagnesium bromide in diethyl ether;hexane;isopropyl alcohol at 0 - 20; for 3 h;Inert atmosphere;
Steps:
1 [Production Example 1] Compound No. 1 1 (Compound in which X is (X-1) in the general formula (1))
nder an argon gas atmosphere, 16.1 g of hexamethyldisilazane and 73.6 g of dehydrated diethyl ether were charged in a 500 mL four-neck reaction flask and cooled with dry ice. Therein, 43.8 g of n-butyllithium (1.6 mol / L) in hexane was added dropwise to react. After completion of the dropwise addition, the temperature was returned to room temperature and the reaction was continued for about 2 hours. Subsequently, the reaction flask was again cooled to -70 ° C. by dry ice and isopropanol, and 13.6 g of phosphorus trichloride was added dropwise to the white suspension for reaction. As the temperature of the reaction solution increased to 0 ° C., the reaction solution became a pale yellow, white suspension from the yellow suspension, and the reaction was continued at 0 ° C. for 1 hour. Then, methyl magnesium bromide (3 mol / L ) In diethyl ether was added dropwise to the reaction mixture. After completion of the dropwise addition, the mixture was stirred at 0 ° C. for 2 hours, and further stirred at room temperature for 1 hour. Filtration was carried out with a membrane filter of 0.5 μm, and diethyl ether and hexane were removed from the obtained filtrate to obtain a liquid residue. The liquid residue was distilled at a bath temperature of 75 ° C. under a reduced pressure of 3.5 Torr to obtain a compound distilled at a column top temperature of 44 ° C. The recovery by this purification was 54%. The obtained compound is subjected to normal temperature and normal pressure (25 ° C., 1 atm, the same applies hereinafter)) And was a colorless transparent liquid. As a result of elemental analysis and 1 H-NMR analysis, the obtained compound was identified as Compound No. 1. It was identified as 1. The results of these analyzes are shown below. The results of TG-DTA are also shown below.
References:
JP2015/54853,2015,A Location in patent:Paragraph 0056
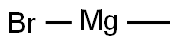
![Phosphorus, [N-ethylmethanethioamidato(2-)-κC]dimethyl[1,1,1-trimethyl-N-(trimethylsilyl)silanaminato]-, (T-4)-](/CAS/20211123/GIF/1236036-93-3.gif)