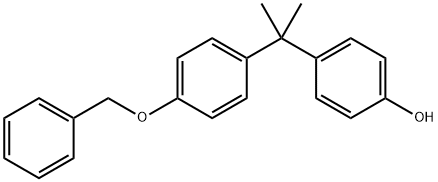
Bisphenol A Monobenzyl Ether synthesis
- Product Name:Bisphenol A Monobenzyl Ether
- CAS Number:42781-88-4
- Molecular formula:C22H22O2
- Molecular Weight:318.41
80-05-7
708 suppliers
$13.00/100g

100-39-0
431 suppliers
$10.00/10g

42781-88-4
14 suppliers
$109.90/5mg
Yield:42781-88-4 59.7%
Reaction Conditions:
with sodium hydroxide in water at 80; for 2 h;Inert atmosphere;
Steps:
4.3 Synthesis of benzyl-protected bisphenol A (1) [33,34]
Bisphenol A (20mmol, 4.566g) was added to a 250mL round bottom flask containing NaOH aqueous solution (40mmol, 150mL, 1.599g). The reactor was then equipped with a reflux condenser and brought to 80°C in a nitrogen environment with vigorous stirring via a Teflon-coated magnetic stir bar. Once completely dissolved, benzyl bromide (20mmol, 0.342g) was added via syringe. The mixture was allowed to stir for 2h at 80°C in a nitrogen environment. After stirring was ceased, a beige precipitate settled out in a clear solution. While still hot, the supernatant was decanted and the crude product washed with water (ca. 80°C). The crude product was then dissolved in chloroform, transferred to a separatory funnel, washed w/10% HCl (v/v), and then washed twice with DI H2O. The organic layer was then dried over magnesium sulfate, filtered into a tared receiver flask, and isolated under vacuum as an off-white solid. The compound was then recrystallized twice from hexanes/ethyl acetate to afford a white, crystalline solid (3.80g, 59.7% yield, mp (DSC)=109°C). ATR-FTIR (neat, cm-1): 3170, 3080, 3030, 2960, 2910, 2860, 1610, 1600, 1580, 1510, 1450, 1380, 1300, 1230, 1180, 1010, 827, 742, 696, 553. 1H NMR (δ, ppm, 500MHz, acetone-d6): 1.60 (s, C(CH3)2, 6H), 5.08 (s, -CH2Ph, 2H), 6.74 (d, 2H, 3JHH=8.7Hz), 6.91 (d, 2H, 3JHH=8.8Hz), 7.06 (d, 2H, 3JHH=8.7Hz), 7.16 (d, 2H, 3JHH=8.8Hz), 7.3-7.5 (m, 5H), 8.08 (s, OH, 1H). 13C (δ, ppm, 125MHz, DMSO-d6): 31.3, 41.5, 69.6, 114.5, 115.1, 127.8, 127.9, 128.1, 128.2, 128.9, 137.7, 141.2, 143.6, 155.4, 155.5.
References:
Campos, Raymond;Mansur, Aleksander A.;Cook, Chloe H.;Batchelor, Benjamin;Iacono, Scott T.;Smith Jr., Dennis W. [Journal of Fluorine Chemistry,2014,vol. 166,p. 60 - 68]