BMS-794833 synthesis
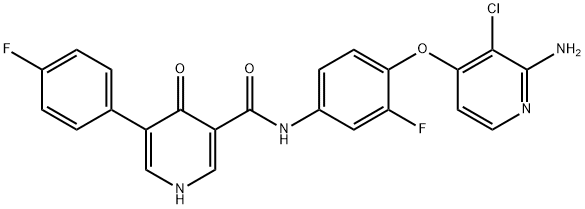
- Product Name:BMS-794833
- CAS Number:1174046-72-0
- Molecular formula:C23H15ClF2N4O3
- Molecular Weight:468.84
![3-Pyridinecarboxamide, N-[4-[[3-chloro-2-[(diphenylmethylene)amino]-4-pyridinyl]oxy]-3-fluorophenyl]-5-(4-fluorophenyl)-1,4-dihydro-4-oxo-](/CAS/20200611/GIF/1174047-03-0.gif)
1174047-03-0

1174046-72-0
General procedure for the synthesis of N-(4-((2-amino-3-chloropyridin-4-yl)oxy)-3-fluorophenyl)-5-(4-fluorophenyl)-4-oxo-1,4-dihydropyridine-3-carboxamide from compound (CAS:1174047-03-0): at room temperature, N-(4-(3-chloro-2-(diphenylmethyleneamino)pyridin-4-yl oxy)-3-fluorophenyl)-5-(4-fluorophenyl)-4-oxo-1,4-dihydropyridine-3-carboxamide (410 mg, 0.65 mmol) solution in THF (10 mL) was slowly added to 2M HCl aqueous solution (0.81 mL, 1.62 mmol). The reaction mixture was stirred at room temperature for 1 hour and then concentrated under vacuum. The residue was cooled to room temperature and 5% aqueous cold hydrochloric acid solution (5 mL) was added. The precipitated solid was collected by filtration, washed sequentially with water and ether and finally dried under vacuum to give the target product (275 mg, 90% yield). The product was confirmed by 1H NMR (DMSO-d6) and mass spectrometry (ESI+): 1H NMR (DMSO-d6) δ 13.31 (s, 1H), 12.70 (br s, 1H), 8.63 (d, 1H, J = 1.30 Hz), 8.09 (d, 1H, J = 1.50 Hz), 8.02 (dd, 1H, J = 2.50, 13.10 Hz), 7.76 (d, 1H, J = 5.50 Hz), 7.71 (m, 2H), 7.44 (dd, 1H, J = 1.50, 8.80 Hz), 7.31 (t, 1H, J = 8.80 Hz), 7.27 (t, 2H, J = 8.80 Hz), 6.43 (br s, 2H), 5.96 (d, 1H, J = 5.60 Hz); MS (ESI+) m/z 469 (M + H)+.
![3-Pyridinecarboxamide, N-[4-[[3-chloro-2-[(diphenylmethylene)amino]-4-pyridinyl]oxy]-3-fluorophenyl]-5-(4-fluorophenyl)-1,4-dihydro-4-oxo-](/CAS/20200611/GIF/1174047-03-0.gif)
1174047-03-0
1 suppliers
inquiry

1174046-72-0
105 suppliers
$28.00/1unit
Yield: 90%
Reaction Conditions:
Stage #1:N-(4-(3-chloro-2-(diphenylmethyleneamino)pyridin-4-yloxy)-3-fluorophenyl)-5-(4-fluorophenyl)-4-oxo-1,4-dihydropyridine-3-carboxamide with hydrogenchloride;water in tetrahydrofuran at 20; for 1 h;
Stage #2: with sodium hydrogencarbonate in waterCooling;
Steps:
1
To a solution of N-(4-(3-chloro-2-(diphenylmethyleneamino)pyridin-4- yloxy)-3-fluorophenyl)-5-(4-fluorophenyl)-4-oxo-l,4-dihydropyridine-3-carboxamide (410 mg, 0.65 mmol) in THF (10 mL) at room temperature was added aqueous HCl (2 M, 0.81 mL, 1.62 mmol). The reaction mixture was stirred at room temperature for 1 h and then concentrated in vacuo. Cold 5% aq. νaHCθ3 (5 mL) was then added to the residue. The solid that formed was collected by filtration, washed with water and then ether, and dried under vacuum to give the desired product (275 mg, 90%). 1H NMR (DMSO-(Z6) δ 13.31 (s, 1 H), 12.70 (br s, 1 H), 8.63 (d, 1 H, J= 1.30 Hz), 8.09 (d, 1 H, J= 1.50 Hz), 8.02 (dd, I H, J= 2.50, 13.10 Hz), 7.76 (d, 1 H, J= 5.50 Hz), 7.71 (m, 2 H), 7.44 (dd, 1 H, J= 1.50, 8.80 Hz), 7.31 (t, 1 H, J= 8.80 Hz), 7.27 (t, 2 H, J= 8.80 Hz), 6.43 (br s, 2 H), 5.96 (d, 1 H, J= 5.60 Hz); MS(ESI+) m/z 469 (M + H)+.
References:
BRISTOL-MYERS SQUIBB COMPANY WO2009/94417, 2009, A1 Location in patent:Page/Page column 27; 37