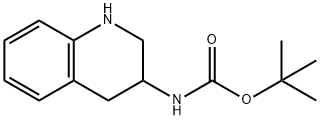
BOC-3-AMINO-1,2,3,4-TETRAHYDROQUINOLINE synthesis
- Product Name:BOC-3-AMINO-1,2,3,4-TETRAHYDROQUINOLINE
- CAS Number:219862-14-3
- Molecular formula:C14H20N2O2
- Molecular Weight:248.32

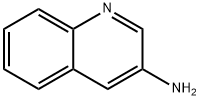
219862-13-2

219862-14-3
The general procedure for the synthesis of tert-butyl (1,2,3,4-tetrahydroquinolin-3-yl)carbamate from the compound (CAS:219862-13-2) is as follows: to a solution of MeOH (150 mL) of 3-quinolinylcarbamic acid was added a solution of MeOH (18 mL) of 1,1-dimethylethyl ester (6.0 g, 24.56 mmol). The reaction mixture was deoxygenated by argon bubbling for 15 minutes and then palladium hydroxide (20% palladium/carbon, 1.2 g) was added. The resulting suspension was subjected to hydrogenation under 45 psi hydrogen pressure for 16 h. Upon completion of the reaction, the catalyst was removed by filtration. The filtrate was concentrated and the residue was dissolved in dichloromethane (CH2Cl2). The resulting dichloromethane solution was washed sequentially with saturated aqueous sodium bicarbonate (NaHCO3) and saturated aqueous sodium chloride (NaCl), dried over anhydrous sodium sulfate (Na2SO4) and concentrated. Finally, the residue was purified by silica gel column chromatography with gradient elution (0 to 50%) of ethyl acetate (EtOAc) in hexane to afford the target product 1A (4.6 g, 75% yield) as a white solid.

219862-13-2
1 suppliers
inquiry

219862-14-3
59 suppliers
$42.00/100mg
Yield: 75%
Reaction Conditions:
with hydrogen;acetic acid;palladium hydroxide on carbon in methanol under 2327.23 Torr; for 16 h;
Steps:
1.A 1A. tert-Butyl 1,2,3,4-tetrahydroquinolin-3-yl-carbamate
To a solution of 3-quinolinylcarbamic acid, 1,1-dimethylethyl ester (6.0 g, 24.56 mmol) in MeOH (150 mL) was added acetic acid (18 mL). The mixture was bubbled with argon for 15 min, then palladium hydroxide (20 weight % palladium on carbon) (1.2 g) was added. The resulting suspension was subjected to hydrogenation under 45 psi of pressure for 16 h, then filtered. The filtrate was concentrated and the residue taken in CH2Cl2. The resulting CH2Cl2 solution was washed with saturated aqueous NaHCO3, saturated aqueous NaCl, dried (Na2SO4) and concentrated. The residue was chromatographed (silica gel) eluting with EtOAc (0 to 50%) in hexanes to give 1A (4.6 g, 75% yield) as a white solid.
References:
Sun, Chongqing;Ewing, William R.;Huang, Yanting;Pendri, Annapurna;Gerritz, Samuel;Ellsworth, Bruce A.;Murugesan, Natesan US2006/160850, 2006, A1 Location in patent:Page/Page column 9
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

219862-14-3
59 suppliers
$42.00/100mg
580-17-6
202 suppliers
$13.43/1gm:

219862-14-3
59 suppliers
$42.00/100mg

24424-99-5
868 suppliers
$13.50/25G

219862-14-3
59 suppliers
$42.00/100mg