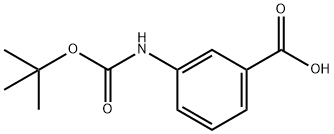
BOC-3-AMINOBENZOIC ACID synthesis
- Product Name:BOC-3-AMINOBENZOIC ACID
- CAS Number:111331-82-9
- Molecular formula:C12H15NO4
- Molecular Weight:237.25

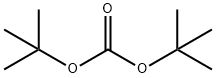
24424-99-5
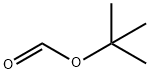
99-05-8

111331-82-9
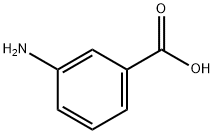
GENERAL METHOD: To a stirred solution of m-aminobenzoic acid (5.00 g, 36.5 mmol) and sodium hydroxide (1.56 g, 39.3 mmol) in a solvent mixture of 1:1 water/dioxane (60 mL) at 0 °C was slowly added di-tert-butyl dicarbonate (14.3 g, 65.4 mmol). The reaction mixture was continued to be stirred at 0 °C for 3 h, followed by slow warming to room temperature and stirring overnight. After completion of the reaction, the reaction mixture was extracted with ethyl acetate (60 mL) and the extraction was repeated once (60 mL). The organic phases were combined and neutralized with 1 M aqueous KHSO4 to pH ≈ 7. The organic layer was separated, washed with water (60 mL) and dried over anhydrous magnesium sulfate. The solvent was removed by concentration under reduced pressure to give 3-(N-Boc-amino)benzoic acid (7.53 g, 87% yield) as an off-white solid, which could be used in the next reaction without further purification.
![tert-butyl [3-(ethoxycarbonyl)phenyl]carbamate](/CAS/20210111/GIF/344462-84-6.gif)
344462-84-6
2 suppliers
inquiry

111331-82-9
133 suppliers
$8.00/1g
Yield: 86%
Reaction Conditions:
Stage #1:tert-butyl [3-(ethoxycarbonyl)phenyl]carbamate with lithium hydroxide;water in tetrahydrofuran;methanol at 20; for 16 h;
Stage #2: with hydrogenchloride in water
Steps:
27
The ester prepared in preparation 25 (750 mg, 2.83 mmol), lithium hydroxide (415 mg, 9.89 mmol), methanol (15 mL), water (15 mL), and tetrahydrofuran (45 mL), were combined in a 250 mL round bottomed flask, fitted with a stirbar, and under a nitrogen atmosphere. The mixture was stirred vigorously at room temperature for 16 hours, then was concentrated under vacuum, dissolved in 1N HCl, and extracted with methylene chloride (100 mL*3). Organic layer was dried (MgSO4), filtered, and concentrated under vacuum, yielding 600 mg of white solid. This material was purified by recrystallization in hexanes:ethyl acetate, yielding the title compound as 580 mg (86%) white solid.[0184] Electrospray-MS 238.0 (M*+1).
References:
Eli Lilly and Company US6639107, 2003, B1 Location in patent:Page/Page column 37

24424-99-5
871 suppliers
$13.50/25G

99-05-8
543 suppliers
$14.00/5g

111331-82-9
133 suppliers
$8.00/1g

99-05-8
543 suppliers
$14.00/5g

111331-82-9
133 suppliers
$8.00/1g