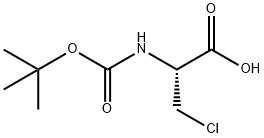
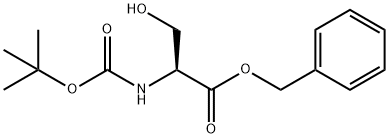
BOC-BETA-CHLORO-ALA-OH synthesis
- Product Name:BOC-BETA-CHLORO-ALA-OH
- CAS Number:71404-98-3
- Molecular formula:C8H14ClNO4
- Molecular Weight:223.65
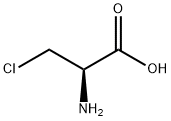
2731-73-9
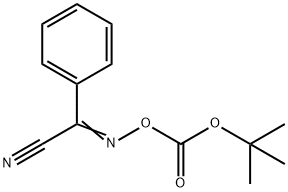
58632-95-4

71404-98-3
GENERAL STEPS: Example 1 Preparation of N-(tert-butoxycarbonyl)-3-chloro-L-alanine To a 50% aqueous 1,4-dioxane solution containing 1.0 g (6.25 mmol) of 3-chloro-L-alanine and 1.3 mL of triethylamine, 1.69 g (6.90 mmol) of 2-(tert-butoxycarbonyloxyimino)-2-phenylacetonitrile was slowly added under stirring at room temperature. After the reaction lasted for 2 hours, the reaction mixture was poured into 20 mL of water and extracted twice with ethyl acetate. The organic layers were combined and washed sequentially with water twice and saturated brine once, followed by drying with anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure to give 0.66 g (40.5% yield) of white solid product. For further purification, recrystallization was carried out using a solvent mixture of ethyl acetate and n-hexane to give 0.58 g of pure product (35.6% yield) with a melting point of 123-124 °C (decomposition). The structure of the product was confirmed by IR and NMR analysis.

2731-73-9
136 suppliers
$45.00/100mg

58632-95-4
277 suppliers
$7.00/5g

71404-98-3
58 suppliers
$26.00/250mg
Yield: 35.6%
Reaction Conditions:
with triethylamine in aqueous 1,4-dioxane;water
Steps:
1 Preparation of N-(tert-Butoxycarbonyl)-3-chloro-L-ala-nine
EXAMPLE 1 Preparation of N-(tert-Butoxycarbonyl)-3-chloro-L-ala-nine To a solution of 1.0 g (6.25 mmol) of 3-chloro-L-alanine and 1.3 mL of triethylamine in 50% aqueous 1,4-dioxane were added 1.69 g (6.90 mmol) of 2-(tert-butoxycarbonyloxyimino)-2-phenylacetonitrile with stirring at room temperature. After 2 hours, the reaction mixture was poured into 20 mL of water and extracted twice with ethyl acetate. The organic layer was washed twice with water and then with saturated brine, dried over anhydrous magnesium sulfate and evaporated to give 0.66 g (40.5%) of white solid. Recrystallization from a mixture of ethyl acetate and n-hexane afforded 0.58 g of the pure product (35.6%) melting at 123-124° C., with decomposition, and having the structural formula: STR72 The identity of the product was confirmed by IR and NMR analyses.
References:
University of Florida US4888427, 1989, A

2731-73-9
136 suppliers
$45.00/100mg

24424-99-5
857 suppliers
$13.50/25G

71404-98-3
58 suppliers
$26.00/250mg
3262-72-4
401 suppliers
$10.00/1g

71404-98-3
58 suppliers
$26.00/250mg
59524-02-6
186 suppliers
$5.00/1g

71404-98-3
58 suppliers
$26.00/250mg