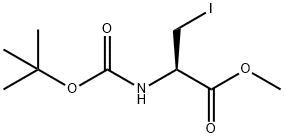
Boc-Beta-iodo-Ala-Ome synthesis
- Product Name:Boc-Beta-iodo-Ala-Ome
- CAS Number:93267-04-0
- Molecular formula:C9H16INO4
- Molecular Weight:329.13
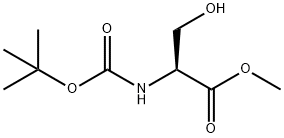
2766-43-0

93267-04-0
General procedure for the synthesis of methyl (R)-2-((tert-butoxycarbonyl)amino)-3-iodopropionate from methyl Boc-L-serinate: a mixture of triphenylphosphine (131 g, 0.500 mol) and imidazole (34 g, 0.50 mol) in dichloromethane (600 mL) was cooled to 0 °C and over 0.5 h iodine was added in batches (127 g, 0.50 mol). The cooling bath was removed and the mixture was stirred for 0.5 hours. After cooling the mixture to 0 °C again, a solution of methyl (R)-2-((tert-butoxycarbonyl)amino)-3-hydroxypropionate (73 g, 0.33 mol) in dichloromethane (300 mL) was slowly added dropwise. After dropwise addition, the cooling bath was removed and the mixture was warmed to room temperature and stirred for 1.5 hours. The reaction mixture was filtered and the filtrate was concentrated to remove most of the solvent. Methyl tert-butyl ether (400 mL) was added to the residue and filtered to remove triphenylphosphine oxide. The filtrate was concentrated and the residue was purified by rapid column chromatography on silica gel to afford methyl (R)-2-((tert-butoxycarbonyl)amino)-3-iodopropionate (74.0 g, 68% yield) as a colorless solid.
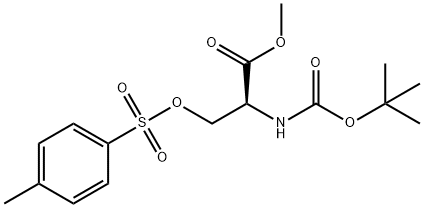
56926-94-4
107 suppliers
$7.00/250mg

93267-04-0
259 suppliers
$5.00/250mg
Yield:93267-04-0 80%
Reaction Conditions:
with sodium iodide in acetone for 22 h;Inert atmosphere;
Steps:
2.2.1; 2.2.2; 2.2.3 Example 2.1 - Step 2: Lab Scale
The entire following procedure was performed in the dark. A 2-liter round bottom flask covered in foil was charged with the tosylate of BOC-L-serine methyl ester 2a (0.40 mol) and acetone (855 mL) under an atmosphere of nitrogen. While stirring, sodium iodide (1.0 mol) was added in one portion and allowed to stir for 22 h, at which time the reaction was complete by thin layer chromatography (30% ethyl acetate in heptane, KMnC stain, disappearance of 2a). The reaction mixture was added slowly over 1 h to 0-5 °C water (3 L) and stirred for 2 h at that temperature. The crude solids were filtered using a fritted funnel, washed with water (3x50 mL), then deliquored for 16 h. A 1-liter round bottom flask was charged with the crude solids and heptane (225 mL). The mixture was warmed to 35-40 °C at which point all of the solids had dissolved. A phase separation was performed and the lower aqueous layer was drained and discarded. The organic layer was cooled to -15 °C and stirred for 2 h. The solids were filtered using a fritted funnel and rinsed twice with the filtrate, then with clean, 0 °C, heptane (20 mL). The solids were dried in a vacuum drying oven at ambient temperature for 24 h, providing pure iodo- intermediate 3a in 80% yield.
References:
VISTAGEN THERAPEUTICS, INC.;LEVIN, Daniel;LEEMING, Peter;EISENREICH, Emerich;LIU, Xuejun, Karl WO2019/157426, 2019, A1 Location in patent:Paragraph 70-91

2766-43-0
308 suppliers
$10.00/1g

93267-04-0
259 suppliers
$5.00/250mg

2766-43-0
308 suppliers
$10.00/1g
55477-80-0
110 suppliers
$130.00/100mg

93267-04-0
259 suppliers
$5.00/250mg

24424-99-5
869 suppliers
$13.50/25G

93267-04-0
259 suppliers
$5.00/250mg
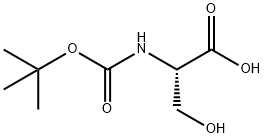
3262-72-4
403 suppliers
$10.00/1g

93267-04-0
259 suppliers
$5.00/250mg