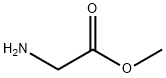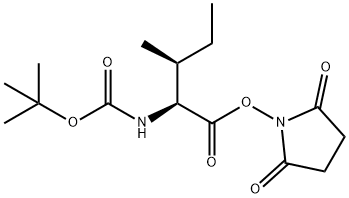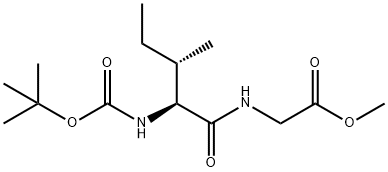
BOC-ILE-GLY-OME synthesis
- Product Name:BOC-ILE-GLY-OME
- CAS Number:16257-04-8
- Molecular formula:C14H26N2O5
- Molecular Weight:302.37
Yield:16257-04-8 91%
Reaction Conditions:
with 1-hydroxy-7-aza-benzotriazole;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;N-ethyl-N,N-diisopropylamine in dichloromethane at 20; for 5 h;
Steps:
Boc-L-Ile-Gly-OH (22).
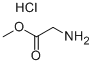
To a solution of glycine methyl ester hydrochloride 18 (1.5 g, 11.9 mmol),Boc-L-Ile-OH (2.3 g, 9.9 mmol), DIPEA (5.2 mL, 29.8 mmol), and HOAt (1.4 g, 10.4 mmol) in CH2Cl2(40 mL) was added EDCHCl (3.8 g, 19.9 mmol) at room temperature. After stirring for 5 h, the reactionmixture was quenched with 1N HCl and extracted with CH2Cl2. The combined organic layer waswashed with aqueous NaHCO3, dried over MgSO4, and concentrated in vacuo. The residue waspurified by flash column chromatography (EtOAc/Hexane = 1:2 to 1:1) to give 2.7 g (91%) of dipeptide 20 as a white solid. [αδ]D20 = -5.85 (c 1.00, CHCl3); 1H-NMR (800 MHz, CDCl3) 6.81 (s, 1H), 5.17 (d,J = 7.5 Hz, 1H), 4.08-3.91 (m, 3H), 3.70 (s, 3H), 1.84 (s, 1H), 1.51-1.45 (m, 1H), 1.39 (s, 9H), 1.13-1.06(m, 1H), 0.91 (d, J = 6.9 Hz, 3H), 0.86 (t, J = 7.4 Hz, 3H); 13C-NMR (200 MHz, CDCl3) 172.1, 170.1, 155.8, 79.9, 59.0, 52.2, 41.0, 37.2, 28.2, 24.6, 15.4, 11.3; IR (thin film, neat) νmax 3324, 2968, 1759, 1687,1661, 1527, 1367, 1173, 862 cm-1; LR-MS (ESI+) m/z 303 (M + H+); HR-MS (ESI+) calcd for C14H27N2O5(M + H+) 303.1914; found 303.1923.
References:
Lim, Changjin [Molecules,2019,vol. 24,# 19,art. no. 3424]
13139-16-7
339 suppliers
$11.00/5G

16257-04-8
5 suppliers
inquiry
![Carbamic acid, N-[(1S,2S)-1-(fluorocarbonyl)-2-methylbutyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/133010-04-5.gif)