
Boron nitride synthesis
- Product Name:Boron nitride
- CAS Number:10043-11-5
- Molecular formula:BN
- Molecular Weight:24.82
B2O3 + 2NH3 → 2BN + 3H2O
Alternatively, the compound can be prepared by heating boric oxide or boric acid with ammonium chloride or an alkali metal cyanide. Purified product can be obtained by high temperature reaction of boron halide with ammonia:
BCl3 + NH3 → BN + 3HCl
Boron nitride can also be made from the elements by heating boron and nitrogen at red heat.

7664-41-7
519 suppliers
$15.00/100ml
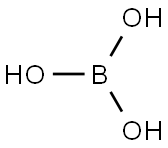
11113-50-1
294 suppliers
$157.00/500g

10043-11-5
370 suppliers
$44.42/50g
Yield:10043-11-5 99%
Reaction Conditions:
with multi-walled carbon nanotubes in solid;byproducts: CO, H2, H2O; mixt. of multi-walled carbon nanotubes and H3BO3 taken in quartz tube, NH3 gas passed through with 10 sccm flow rate at 200 °C 2 h, temp.slowly raised to 1000 °C for 3 h;
References:
Deepak;Vinod;Mukhopadhyay;Govindaraj;Rao [Chemical Physics Letters,2002,vol. 353,# 5-6,p. 345 - 352]

11113-50-1
294 suppliers
$157.00/500g
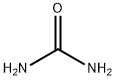
57-13-6
800 suppliers
$5.00/5g

10043-11-5
370 suppliers
$44.42/50g
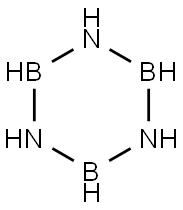
6569-51-3
38 suppliers
inquiry

10043-11-5
370 suppliers
$44.42/50g
78837-91-9
0 suppliers
inquiry

10043-11-5
370 suppliers
$44.42/50g
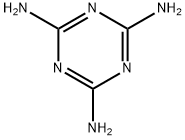
108-78-1
829 suppliers
$5.00/25g

11113-50-1
294 suppliers
$157.00/500g

10043-11-5
370 suppliers
$44.42/50g