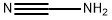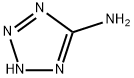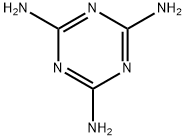
Melamine synthesis
- Product Name:Melamine
- CAS Number:108-78-1
- Molecular formula:C3H6N6
- Molecular Weight:126.12
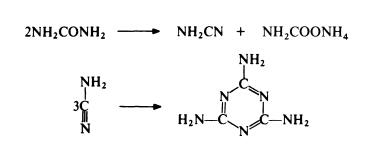
Thus only 50% of the urea used gives melamine in one step and ammonium carbamate has to be separated and converted to urea for recycling. Despite this limitation, the urea route is the most economical of currently available routes.
Yield:108-78-1 93%
Reaction Conditions:
with potassium hydroxide in water monomer;dimethyl sulfoxide;
Steps:
4 EXAMPLE 4
EXAMPLE 4 A solution of 30 g of dicyandiamide and 30 g of solid cyanamide in 120 g of dimethylsulfoxide is added over a period of 11 minutes to a mixture, heated to 180° C, of 4.0 g of potassium hydroxide and 4.0 g of dicyandiamide in 80 g of dimethylsulfoxide. After an additional 5 minutes of reaction time at 180° C the mixture is cooled. The precipitated melamine is removed by filtration, stirred up in water, and again filtered. The first yield amounts to 53.8 g of melamine or 84.1%. As a second yield, an additional 5.7 g of 8.9% of melamine was found in the filtrate and wash water, so that the total yield amounted to 93.0%.
References:
US4069383,1978,A
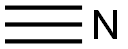
74-90-8
0 suppliers
inquiry

108-78-1
830 suppliers
$5.00/25g
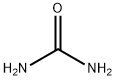
57-13-6
800 suppliers
$5.00/5g

108-78-1
830 suppliers
$5.00/25g
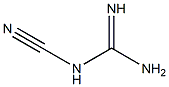
780722-26-1
4 suppliers
inquiry

108-78-1
830 suppliers
$5.00/25g