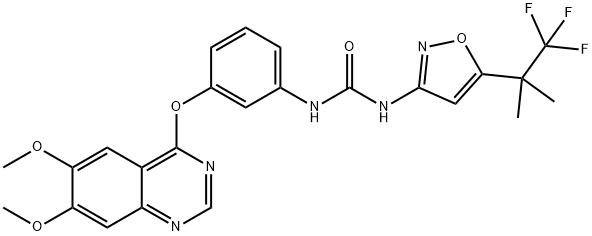
CEP-32496 (free base) synthesis
- Product Name:CEP-32496 (free base)
- CAS Number:1188910-76-0
- Molecular formula:C24H22F3N5O5
- Molecular Weight:517.46
![3-[(6,7-dimethoxy-4-quinazolinyl)oxy]aniline](/CAS/20180629/GIF/1188908-37-3.gif)
1188908-37-3
![[5-(2,2,2-TRIFLUORO-1,1-DIMETHYL-ETHYL)-ISOXAZOL-3-YL]-CARBAMIC ACID PHENYL ESTER](/CAS/GIF/1188911-77-4.gif)
1188911-77-4

1188910-76-0
A 10L Chemglass jacketed reactor, equipped with an N2 inlet/outlet, was charged with 3-[(6,7-dimethoxy-4-quinazolinyl)oxy]aniline (Compound 2, 200.0 g, 637 mmol) and phenyl (5-(1,1,1-trifluoro-2-methylpropan-2-yl)isoxazol-3-yl)carbamate (Compound 3, 177.0 g, 596 mmol). equipped with N2 inlet/outlet, 4-dimethylaminopyridine (DMAP, 2.88 g) and 4.0 L of isopropylacetic acid were added. The internal temperature of the reaction mixture was raised to 70 °C and maintained at this temperature for 9 hours. The slurry was maintained during the reaction and HPLC monitoring showed that compound 2 was completely consumed after a period of heating. Subsequently, 2.0 L of heptane was added at 70 °C and the reaction mixture was cooled to 20 °C. Stirring was continued for 1 h to precipitate the solid, filtered, and the filter cake was washed with 2.0 L of a 1:1 (v/v) isopropyl acetate/heptane solvent mixture. The resulting white solid was dried under vacuum at 55 °C and 75 mbar by N2 exhaust to give 295 g (96% yield) of form A0 product with 99.3% HPLC purity.
![3-[(6,7-dimethoxy-4-quinazolinyl)oxy]aniline](/CAS/20180629/GIF/1188908-37-3.gif)
1188908-37-3
14 suppliers
inquiry
![[5-(2,2,2-TRIFLUORO-1,1-DIMETHYL-ETHYL)-ISOXAZOL-3-YL]-CARBAMIC ACID PHENYL ESTER](/CAS/GIF/1188911-77-4.gif)
1188911-77-4
10 suppliers
inquiry

1188910-76-0
97 suppliers
$35.00/500μg
Yield: 96%
Reaction Conditions:
with dmap in Isopropyl acetate at 70; for 9 h;Inert atmosphere;
Steps:
Preparation from Form A0
To a 10 L Chemglass jacketed reactor with N2 inlet/outlet was added compound 2 (200.0 g, 637 mmol), compound 3 (177.0 g, 596 mmol) and 4-Dimethylaminopyridine (DMAP) (2.88 g) followed by 4.0 L of isopropyl acetate. The internal temperature was raised to 70°C and heated for 9 hours. The reaction remained a slurry throughout there action and HPLC showed no compound 2 remaining after heating for that period. Next,2.0 L of heptane were added at 70°C and the reaction cooled to 20°C. The solids were stirred for lhour, filtered and the cake washed with 2.0 L of 1:1 isopropyl acetate/ heptanes. The white solids were placed in an oven with N2 bleed at 55°C under 75 mbar vacuum. The resulting solids weighed 295 g (96% yield) of Form A0 with 99.3 % purity by HPLC.
References:
CEPHALON, INC.;BIERLMAIER, Stephen J.;HALTIWANGER, Ralph C.;JACOBS, Martin J. WO2014/164648, 2014, A2 Location in patent:Paragraph 23
![3-[(6,7-dimethoxy-4-quinazolinyl)oxy]aniline](/CAS/20180629/GIF/1188908-37-3.gif)
1188908-37-3
14 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1188910-76-0
97 suppliers
$35.00/500μg
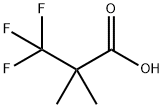
889940-13-0
176 suppliers
$25.00/250mg

1188910-76-0
97 suppliers
$35.00/500μg
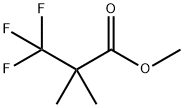
1188911-72-9
79 suppliers
$45.00/50mg

1188910-76-0
97 suppliers
$35.00/500μg
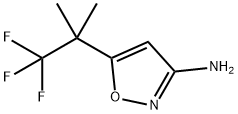
1188911-74-1
35 suppliers
$1680.00/1g

1188910-76-0
97 suppliers
$35.00/500μg