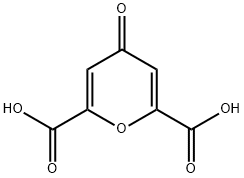
Chelidonic acid synthesis
- Product Name:Chelidonic acid
- CAS Number:99-32-1
- Molecular formula:C7H4O6
- Molecular Weight:184.1
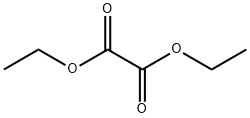
95-92-1

67-64-1

99-32-1
A modified literature method was used to synthesize betulinic acid from diethyl oxalate and acetone. The steps were as follows: in a typical experiment, sodium ethoxide (69.4 g, 1.02 mol) was suspended in ethanol (300 mL). After stirring at 60 °C for 45 min, a mixed solution of anhydrous acetone (29 g, 38 mL, 0.5 mol) and diethyl oxalate (155 g, 144 mL, 1.06 mol) was slowly added dropwise. After the dropwise addition, the reaction temperature was maintained at 60 °C and the reaction continued to be stirred for 14 hours. Subsequently, 37% aqueous hydrochloric acid solution (230 mL) and water (100 mL) were added to the reaction mixture and the reaction was stirred for 24 h. After 24 h, about half of the aqueous ethanol was evaporated under reduced pressure, and water (300 mL) and 37% aqueous hydrochloric acid solution (60 mL) were then added to the remaining mixture, and the stirring was continued at 50 °C for 20 h. The reaction was completed by filtration and the mixture was collected. Upon completion of the reaction, the resulting solid was collected by filtration and washed sequentially with water and acetone. The resulting solid was recrystallized using activated carbon in boiling water. The filtrate was allowed to stand at room temperature until crystals precipitated. The white crystals were collected and dried under vacuum to give the final target product leucocholic acid (42.3 g, 46% yield).
Yield:99-32-1 46%
Reaction Conditions:
Stage #1:oxalic acid diethyl ester;acetone with sodium ethanolate in ethanol at 60; for 15 h;
Stage #2: with hydrogenchloride;water in ethanol for 44 h;
Steps:
13 4.1.2.11 Chelidonic acid (4-oxo-4H-pyran-2,6-dicarboxylic acid) (12)
Chelidonic acid was synthesized using an adapted literature protocol.33 In a typical experiment, EtONa (69.4 g, 1.02 mol) was suspended in ethanol (300 mL). After 45 min at 60°C, a solution of dry acetone (29 g, 38 mL, 0.5 mol) and diethyl oxalate (155 g, 144 mL, 1.06 mol) was added over a period of 1 h. After the addition was complete, the solution was maintained at 60°C and stirred for an additional 14 h. Then, 230 mL of HCl (37% aqueous solution) and 100 mL of water were added and the mixture was stirred for 1 day. After 24 h, about half of aqueous ethanol was removed under reduced pressure then 300 mL of water and 60mL of HCl (37% aqueous solution) were added to this mixture and stirring was continued for 20 h at 50°C. The resulting solid was filtered off, washed first with water then with acetone before recrystallization from boiling water using charcoal. The filtrate was left at room temperature until formation of crystals. The white crystals were collected and dried under vacuum to give the desired compound 12 (42.3 g, 46%).
References:
Gracia, Stéphanie;Arrachart, Guilhem;Marie, Cécile;Chapron, Simon;Miguirditchian, Manuel;Pellet-Rostaing, Stéphane [Tetrahedron,2015,vol. 71,# 33,p. 5321 - 5336]
68854-18-2
16 suppliers
$189.00/250mg

99-32-1
204 suppliers
$9.00/250mg
105-50-0
405 suppliers
$6.00/5g

99-32-1
204 suppliers
$9.00/250mg
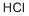
7647-01-0
0 suppliers
$10.00/10g

68854-18-2
16 suppliers
$189.00/250mg

99-32-1
204 suppliers
$9.00/250mg