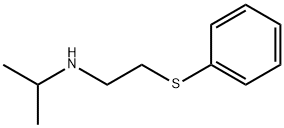
CHEMBRDG-BB 9071613 synthesis
- Product Name:CHEMBRDG-BB 9071613
- CAS Number:67747-26-6
- Molecular formula:C11H17NS
- Molecular Weight:195.32
Yield:67747-26-6 81%
Reaction Conditions:
with tributylphosphine in tetrahydrofuran at 20;Schlenk technique;Inert atmosphere;
Steps:
8.7 (2R,3S)-N-isopropyl-3-(phenylthio)butan-2-amine ((S,R)-L4)
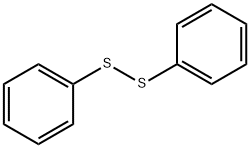
General procedure: To a solution of 6 (200mg, 1.53mmol) in THF (0.8mL), diphenyl disulfide (665mg, 3.05mmol) and tri-n-butylphosphine (616mg, 3.05mmol, 749μL) were added and the reaction mixture was stirred overnight at RT. The solvent was then evaporated, 5mL of water was added and the pH was adjusted to 2 by using dilute HCl solution. The mixture was extracted with ether (20mL) and the pH of the aqueous phase was adjusted to 12 with Na2CO3. The alkaline solution was then extracted with ether (4×20mL) and the solvent was evaporated in vacuo. To remove the phosphine oxide byproduct the crude mixture was passed through a short pad of activated Al2O3 column by using ether as eluent. The evaporation of the solvent gave the pure product as an oil. Yield: 218mg, 64%.
References:
Major, Máté M.;Császár, Zsófia;Bényei, Attila C.;Balogh, Szabolcs;Bakos, József;Farkas, Gergely [Journal of Organometallic Chemistry,2020,vol. 921,art. no. 121332]
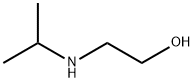
5535-49-9
118 suppliers
$29.00/5g
75-31-0
443 suppliers
$14.00/25mL

67747-26-6
8 suppliers
$46.00/1g